Team:Calgary Entrepreneurial/Project/Manufacturing
From 2013.igem.org



FREDsense's website works best with Javascript enabled, especially on mobile devices. Please enable Javascript for optimal viewing.
Operations and Manufacturing
Another important aspect of our business is how we will manufacture and distribute our product. It is important to consider factors such as cost, location and timeline. Through a partnership with a local manufacturing group, we have established an operational plan that we hope can achieve our production goals at low cost.
Manufacturing
Developing Prototypes and Manufacturing Models
With a technology implementation plan in place we have developed drawings and plans to be able to optimize our prototype system and prepare it as a product for delivery into the market. Below are schematics of potential forms of our system:
While these prototypes are the primary manufacturing layouts that have been tested so far, we have also analyzed potential other means of developing prototypes such as microfluidics. An example of a microfluidic chip layout that could be used is seen below. Microfluidics offers a distinct advantage in terms of price per each unit that must be produced. Because it is on a micro-scale, it uses less of each reagent and component making them more simple to handle and effective to use. Please note, that the numbers reported on this page reflect the cuvette style cartridge.
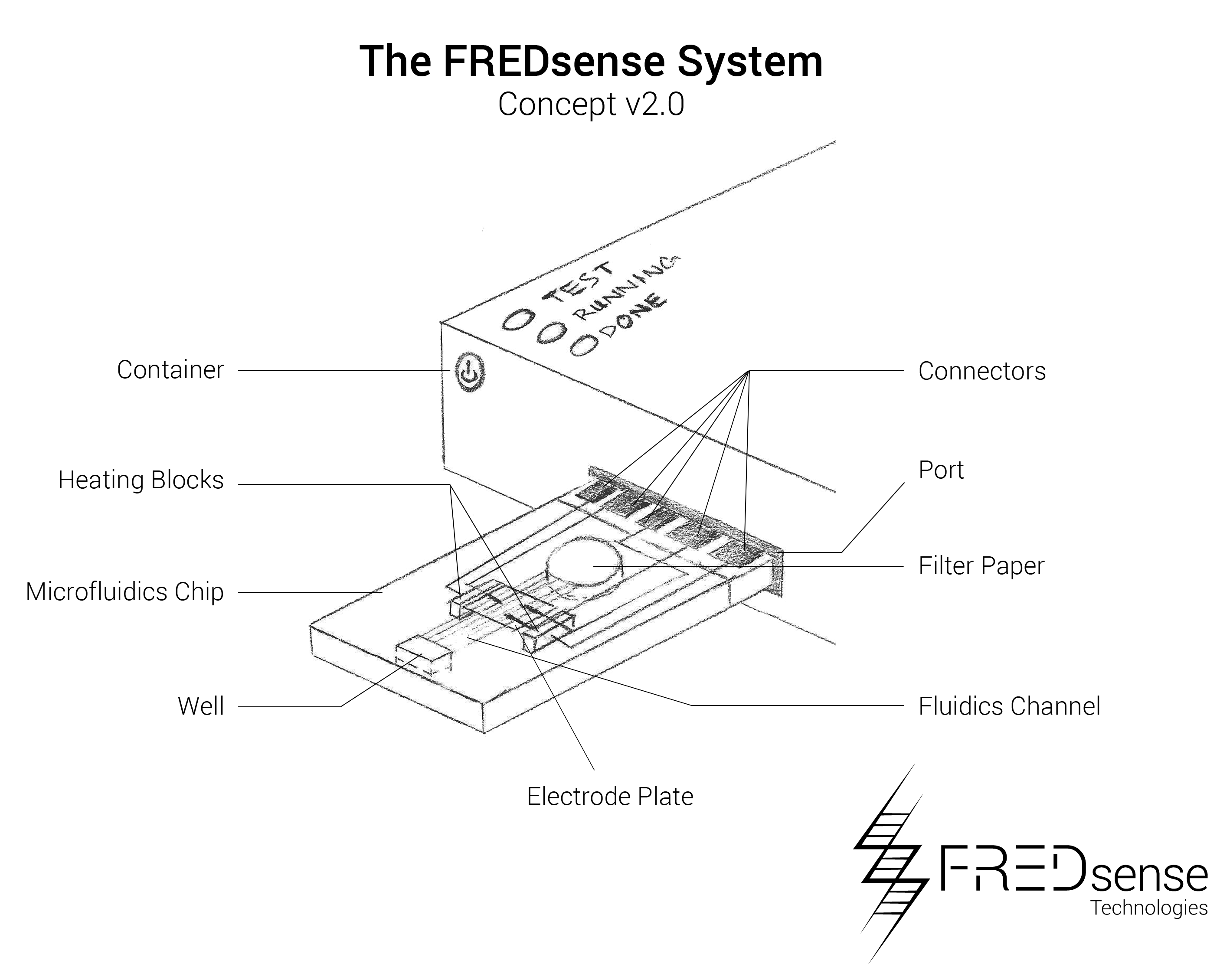
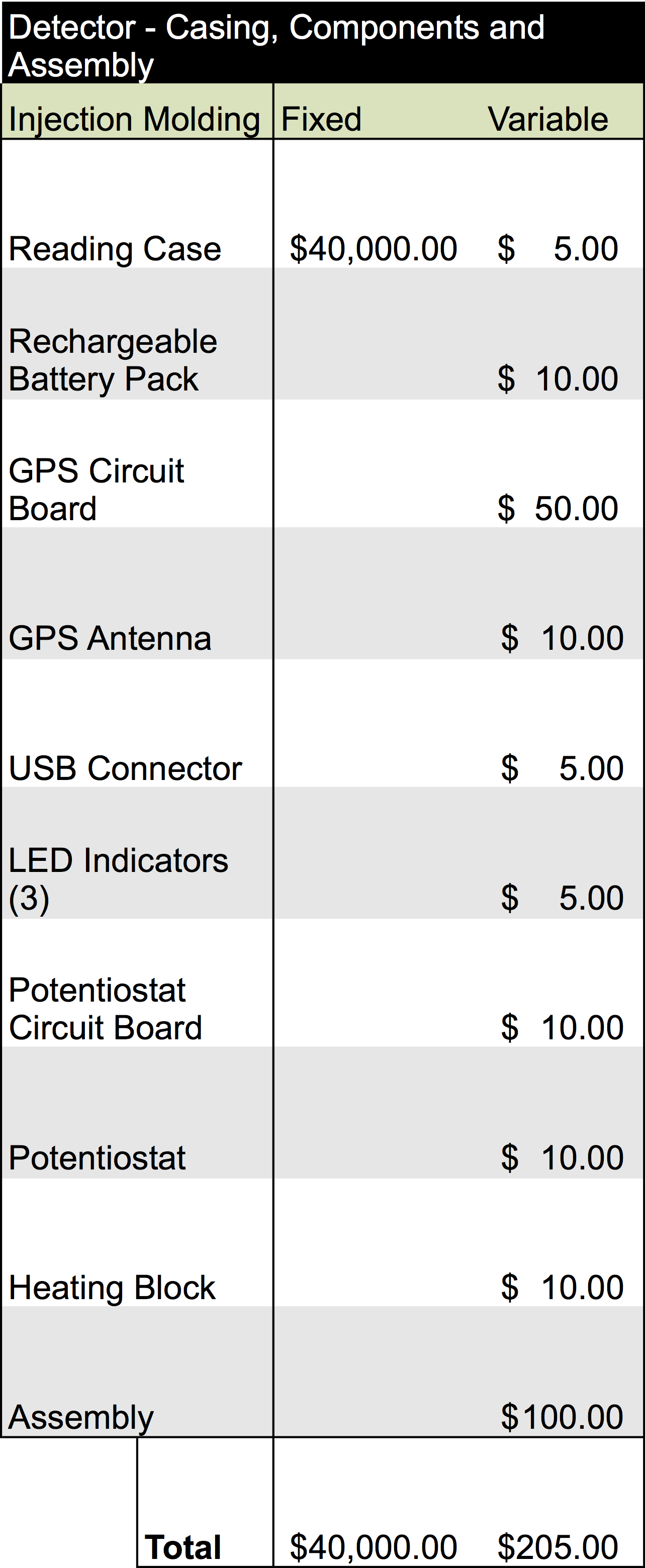
Components
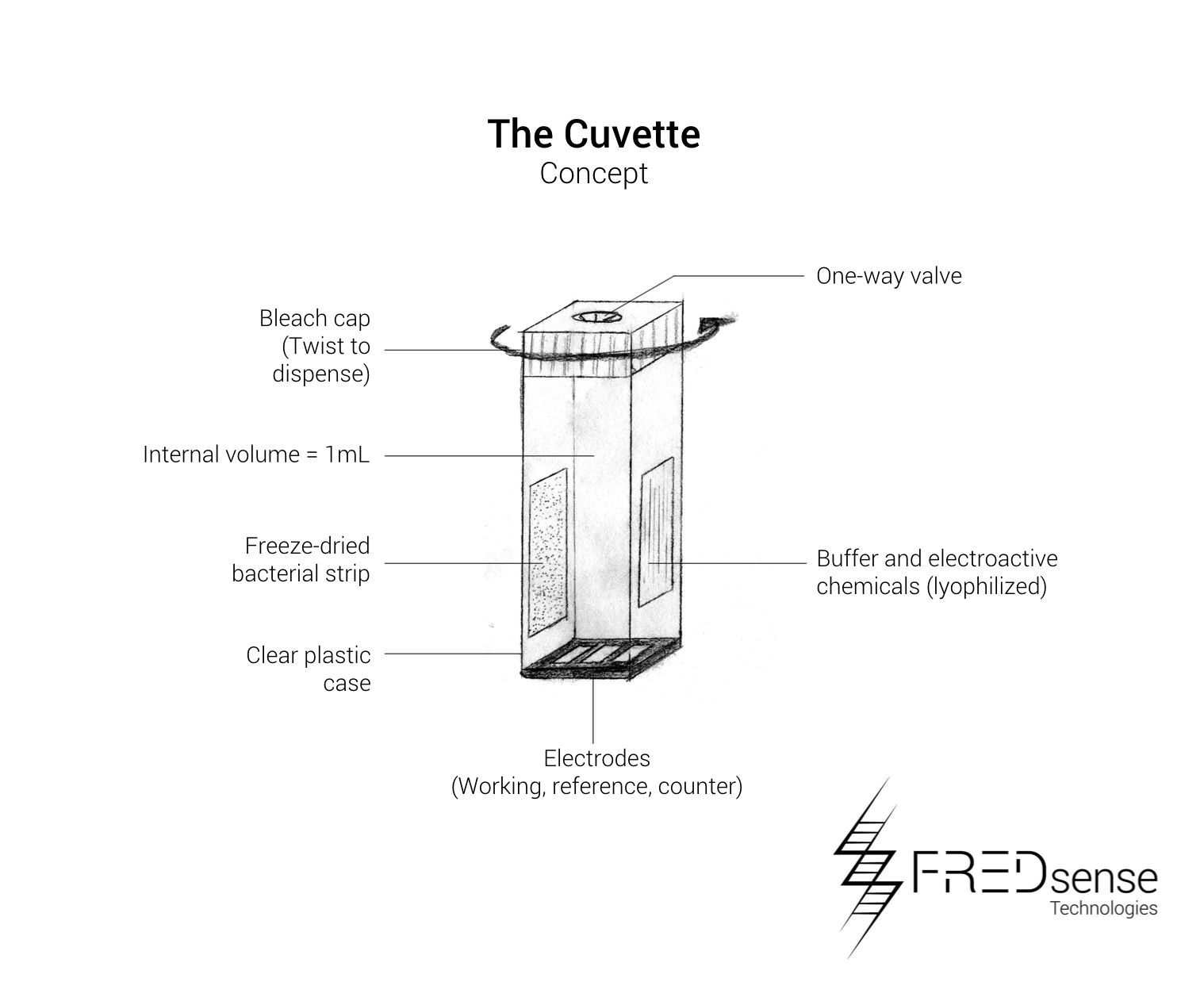
These include the basic components required to build each device. The cartridge is designed to be made of a durable injectable molding plastic holding the water sample solution in a volume of approximately 1 mL giving the cuvette dimensions of about 20 mm x 50 mm x 10 mm each. A one-way valve will ensure the sample is contained within the device, and the capsule will contain a twist tube based cap which will dispense bleach once the cartridge has been finished being used. This will be used as one of our safety features of the device to ensure containment and no release of our organism into the environment
The device has a similar design made out of molded plastic that is rugged and durable to be able to be used in the field. The basic design incorporates a heat source to ensure the electrodes of the cartridges are at a consistent temperature, a potentiostat to do the electrochemical detection, and electronic systems for handling the data that is collected. The LED lights will be used as external indicators to the user if the test was successful or if it should be repeated.
Manufacturing Cost Analysis and Development Timeline
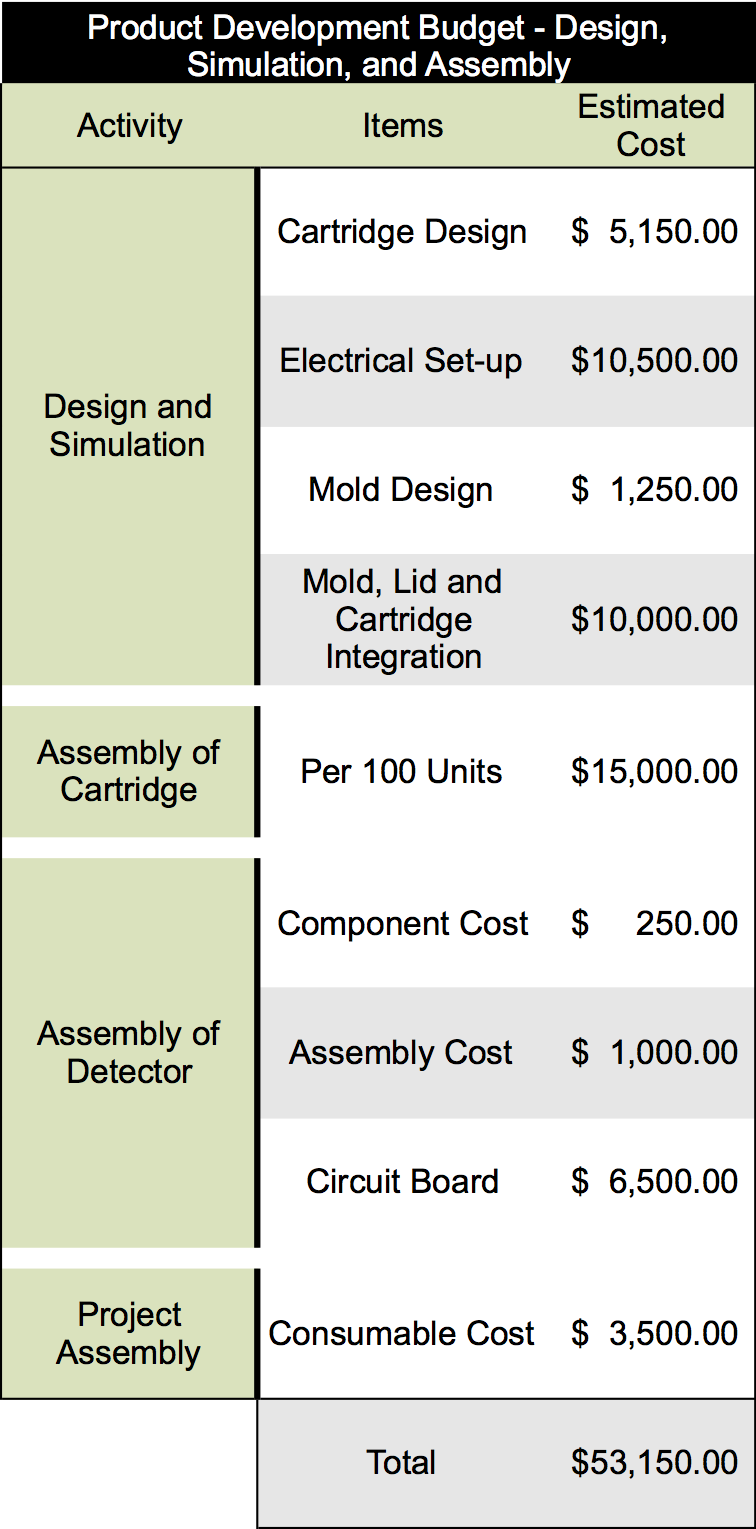
Once a lab-scale prototype has been optimized, we will move our product forward into a manufacturing development phase. To accomplish this task, we will partner with industrialization not-for-profit organizations and government programs that specialize in prototype up-scale and optimization. An overview of the cost estimations that have been made for one round of prototype development are below:
These numbers were determined by estimating the cost of each component of the project and the cost associated with batch producing 100 cartridges. To produce our final product several rounds of prototype and product development will have to be undergone to ensure the production of an effective product. It is estimated that our product development will require at least five rounds of prototype development cycles as compared to other similar products in this industry. Overall we have estimated the cost of these five rounds will be in the range of $225,000 to $275,000 depending on changes that are made to the technology through the development cycle.
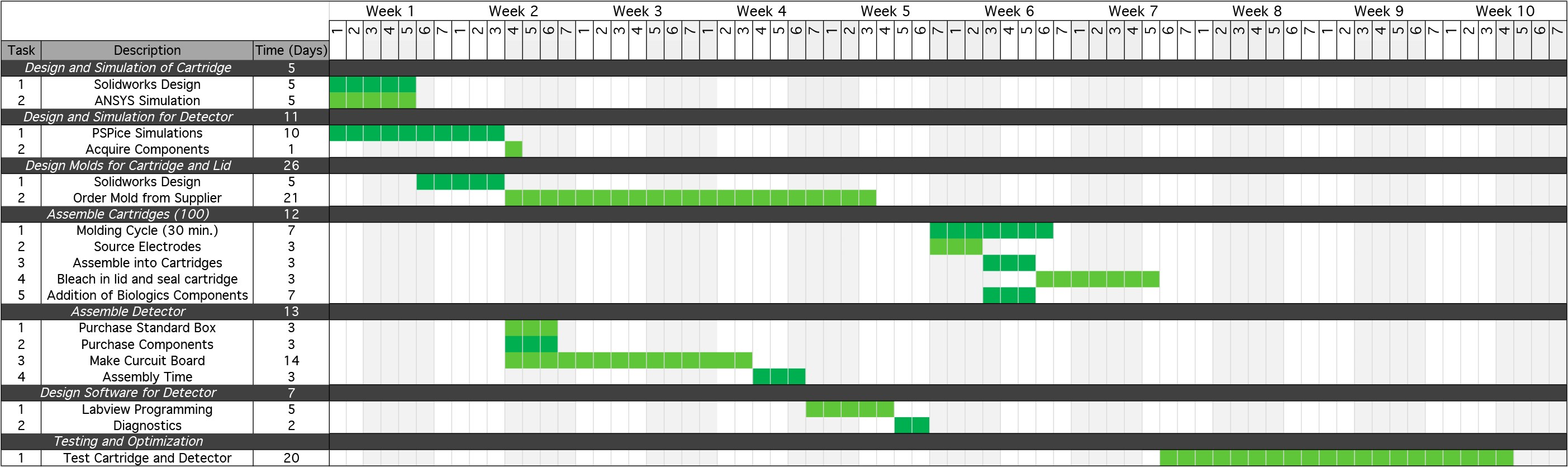
Each round of prototype development will require a series of steps to design and simulate the prototype, design the molding and plastic casings for each device, assemble each component individually, and then integrate the components into the finalized system. An overview of this process and the time associated with each step is listed below:
To perfect our final product, several rounds of prototype and product development will have to be performed. It is estimated that our product development will require at least five rounds of prototype development cycles, which is similar to other products in this industry. Overall we have preliminarily estimated the cost of these five rounds will be in the range of $200,000 to $250,000. This has been determined by collaborating with ACAMP, an Alberta manufacturing not-for-profit organization that aids in development of start-up ventures. Once our finalized product is ready to enter mass production, estimated analysis for the cost of building the device would require an overhead of about $215.00 per unit. Additionally, producing one of the cartridges would cost $5.80 per unit. This is based upon comparable products already being manufactured in this market.Prototype Development Timeline
INSERT PICTURE OF GANTT LIKE CHART SHOWING TIMELINE FOR DEVELOPMENT OVERALL!!!
Cost of Production
INPUT FIGURE FROM DEVELOPMENT CYCLE COST DOCUMENT
Once our finalized product is ready to enter mass production, estimated analysis for the cost of building the device would require an overhead of about $215.00 per unit. Additionally, producing one of the catridges would be equivalent to $5.80 per unit. The information for this analysis is below:
Manufacturing Operations
Production will be facilitated by partnering with key manufacturing services and operation experts. One such example of one of these partners would be ACAMP - the Alberta Center for the Advancement of MNT Products. This government funded organization provides expertise and funding to commercialize products. These key players will be responsible for mass production of the plastics, electrical components, and hardware components for both the catridges and the final detector device. The bacterial systems will be made in-house, lyophilized, and added to the cartridge in one of our own facilities. The amount of manufacturing which is done by our group will be set to a minimum and only include those particular components which must be completed in a biological manufacturing facility.
Starting capacity for operations will be set to accomidate the production of bulk numbers of catridges in the hundreds (involving daily operations) with the intent of increasing this production to accomidate thousands of catridges to be produced. This will be done through further partnerships to diverse manufacturing groups outside of Canada as we begin to move our production to a more global audience.
I DONT KNOW WHAT TO DO FOR STANDARD COSTS OF PRODUCTION
 "
"