Team:CAU China/Data
From 2013.igem.org

|
JanuaryForm our team and find our instructors Registry for a CAU iGEM team. Congratulations~ Brainstorm for our projects Journal clubs for scientific issue FebruaryEngaged in extensive reading of references Drafting team execution rules Design our logo: the first draft is awesome~ (~ o ~) MarchRedesign our logo Discuss our E-Periodicals AprilBrainstorm for how our projects carry out Literature review MayOur first e-periodical comes out~o(≧v≦)o~ Yah ~ Recruit new members Open our project July==== Week 4 July 28th Wet lab Got Nadh1 (adh1 from Neurospora crassa) cDNA from He lab Got Sadh2 (adh2 from Saccharomyces cerevisiae) cDNA from Lou lab Lab meeting Journal club July 29th Wet la bGot expression vector pET-28a(+) from Novagen Preparing LB and LB-Kanamycin plates Autoclave basic materials ====July 30th ====Wet lab Got E.coli DH5α, JM109, BL21(DE3) from Chen lab Order the restriction endonuclease and DNA polymerase Dry labPrimers design for Nadh1, Sadh2 and ta0841 (Thermoplasma acidophilum) AugustWeek 1 August 1st Wet labTransformation of pET-28a(+) for plasmid propagation Digestion of vector with BamHI and SalI PCR for Nadh1 and Sadh2 Gel electrophoresis of digested product and PCR product Plasmid DNA isolation for pET-28a(+) August 2ed Wet labGel purification of digested vector gel product and PCR product Gel check of plasmid extraction Digestion of Sadh2 PCR product with BamHI and SalI Ligation of Sadh2 with pET-28a(+) August 3rd Wet labTransformation of ligated pET-28a(+)-Sadh2 Clone PCR detection for transformation Small inculation of pET-28a(+)-Sadh2(1st) Dry labCalculation of the entropy evolution Week 2 August 4th Wet labDigestion of vector with BamHI and EcoRI Digestion of Nadh1 PCR product with BamHI and EcoRI Gel electrophoresis of digested vector product Gel purification of digested vector gel product Ligation of Nadh1 with pET-28a(+) August 5th Wet labTransformation of ligated pET-28a(+)-Nadh1(1st) Colony PCR identification for transformation
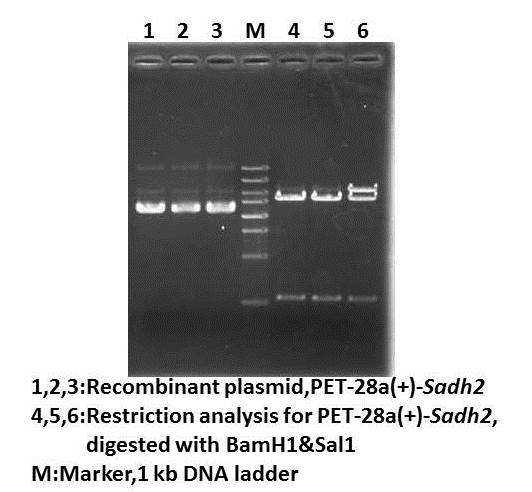
Project interim report Brainstorming sessions for division of work and experiment Dry lab August 7thWet lab Plasmid extraction for pET-28a(+)-Sadh2 Restriction analysis and DNA sequencing of pET-28a(+)-Sadh2 for identification Result:positive!
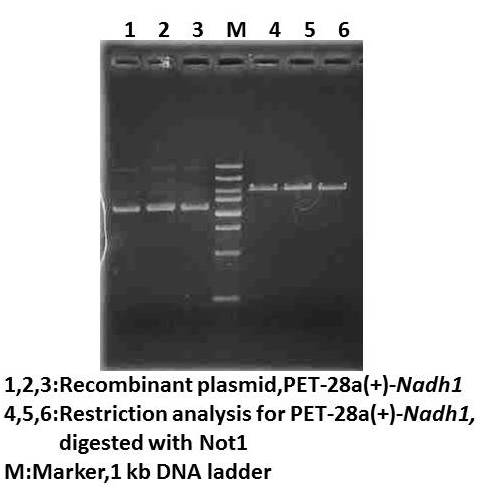
Dry lab August 8thWet lab Ligation of Nadh1 with pET-28a(+) Transformation of ligated pET-28a(+)-Nadh1(2nd) Result:no clone(⊙o⊙)! Dry lab August 9thWet lab Ligation of Nadh1 with pET-28a(+) Transformation of ligated pET-28a(+)-Nadh1(2nd) Result:no clone o_O??? Dry lab August 11thWet lab Discussion for experiment PCR of Nadh1 Gel electrophoresis of PCR product Digestion of vector and PCR product with BamHI and EcoRI (for a longer time) Gel electrophoresis of digested vector product Gel purification of digested vector gel product Column- purification of digested gene product Ligation of Nadh1 with pET-28a(+) August 12thWet lab Transformation of ligated pET-28a(+)-Nadh1(3rd) Colony PCR detection for transformation Result:no positive clone (+﹏+)~ Dry lab Calculation of the entropy evolution in another way August 13thWet lab Discussion for experiment Dry lab August 14thWet lab Inoculation of pET-28a(+): repeat Inoculation Dry lab Primers redesign for Nadh1 August 15thWet lab Clean the lab Preparing IPTG for protein induction Autoclave the materials Dry lab August 16thWet lab Small inoculation for pET-28a(+)-Sadh2 Large inoculation for pET-28a(+)-Sadh2 Protein induction with 0.1mM IPTG, 37℃ Cell lysis Enzyme activity assay(1st) Dry lab August 18thWet lab Informal lab meeting August 19thWet lab Small inoculation for pET-28a(+)-Sadh2 Large inoculation for pET-28a(+)-Sadh2 Protein induction with 0.5mM IPTG ,16℃ Cell lysis SDS-PAGE for protein induced assay Result:no protein induced Dry lab August 20thWet lab Small inoculation for pET-28a(+)-Sadh2 Large inoculation for pET-28a(+)-Sadh2 Protein induction with 0.1mM and 0.5mM IPTG,4℃ Cell lysis SDS-PAGE for protein induced assay Result: protein in precipitation not separate well Dry lab August 21stWet lab SDS-PAGE again for induction yesterday Result:no protein induced in precipitation Dry lab August 22ndWet lab New primers for Nadh1 arrival \(^o^)/YES! PCR for Nadh1 with new primers Gel electrophoresis of PCR product Digestion of vector and PCR product with BamHI and NotI Gel electrophoresis of digested vector product Gel purification of digested vector gel product Column-purification of digested gene product Ligation of Nadh1 with pET-28a(+) Dry lab August 23rdWet lab Transformation of ligated pET-28a(+)-Nadh1(4th) Result:no clone Ligation of Nadh1 with pET-28a(+) Dry lab August 24thWet lab Transformation of ligated pET-28a(+)-Nadh1 (5th) Result:no clone o(︶︿︶)o Dry lab
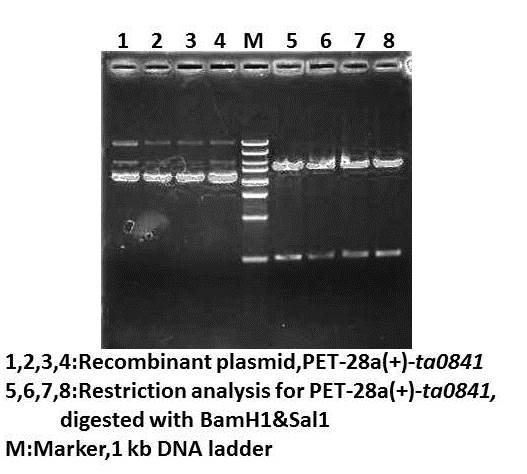
August 25thLab meeting and discuss for experiment August 26thWet lab Get the ta0841 (commercially synthesized CDS, BGI Crop) PCR for ta0841 amplification Gel electrophoresis of PCR product Dry lab August 27thWet lab Digestion of vector and PCR product with BamHI and SalI Gel electrophoresis of digested vector product Gel purification of digested vector gel product Column-purification of digested gene product Ligation of ta0841 with pET-28a(+) Dry lab August 28thWet lab Transformation of ligated pET-28a(+)-ta0841 (1st) Preparing of LB and autoclave LB-Kanamycin, ampicillin and chloramphenicol plates Order restriction endonuclease(NEB) and DNA Marker Result:no colone Dry lab August 29stWet lab Ligation of ta0841 with pET-28a(+) Ligation of Nadh1 with pET-28a(+) Transformation of ligated pET-28a(+)-ta0841 (2nd) and pET-28a(+)-Nadh1 (6st) Dry lab August 30thWet lab Colony PCR for transformation product identification Small inoculation of pET-28a(+)-ta0841 (2st) and pET-28a(+)-Nadh1 (6st) for restriction anlysis Dry lab August 31stWet lab Plasmid extraction from inoculation yesterday Result:none plasmid isolation Supercompetent cell Preparation for E.coli DH5αand JM109 Dry lab SeptemberSeptember 1stLab meeting Discussion for experiment and wiki September 2nd Wet lab PCR for ta0841 and Nadh1 amplification Gel electrophoresis of PCR product Gel purification of PCR product Nanoview: the concentration is OK. \(^o^)/YEH~ Digestion of pET-28a(+) by BamH1-HF and Sal1-HF for ta0841; Not1-HF and BamH1-HF for Nadh1 Nanoview: the concentration is not high (⊙_⊙?) Digestion of ta0841 with BamH1-HF and Sal1-HF Digestion of Nadh1 with Not1-HF and BamH1-HF Column-purification of digested gene Nanoview: the concentration is OK O(∩_∩)O~ Ligation of ta0841 with pET-28a(+) Ligation of Nadh1 with pET-28a(+) Dry lab Building enzyme kinetic equations to describe the enzyme catalysis reactions September 3rdWet lab Transformation of ligated pET-28a(+)-ta0841 (3rd) and pET-28a(+)-Nadh1 (7st) Colony PCR for transformation product identification Gel check for colony PCR Result:positive results! ( ⊙o⊙ ) Small inoculation of pET-28a(+)- (3rd) and pET-28a(+)-Nadh1 (7st) for restriction anlysis Dry lab Building enzyme kinetic equations to describe the enzyme catalysis reactions September 4stWet lab Plasmid extraction of pET-28a(+)-ta0841 (3rd) and pET-28a(+)-Nadh1 (7st) Restriction analysis for identification Result:positive results!!! ~\(≧▽≦)/~ Bravo~
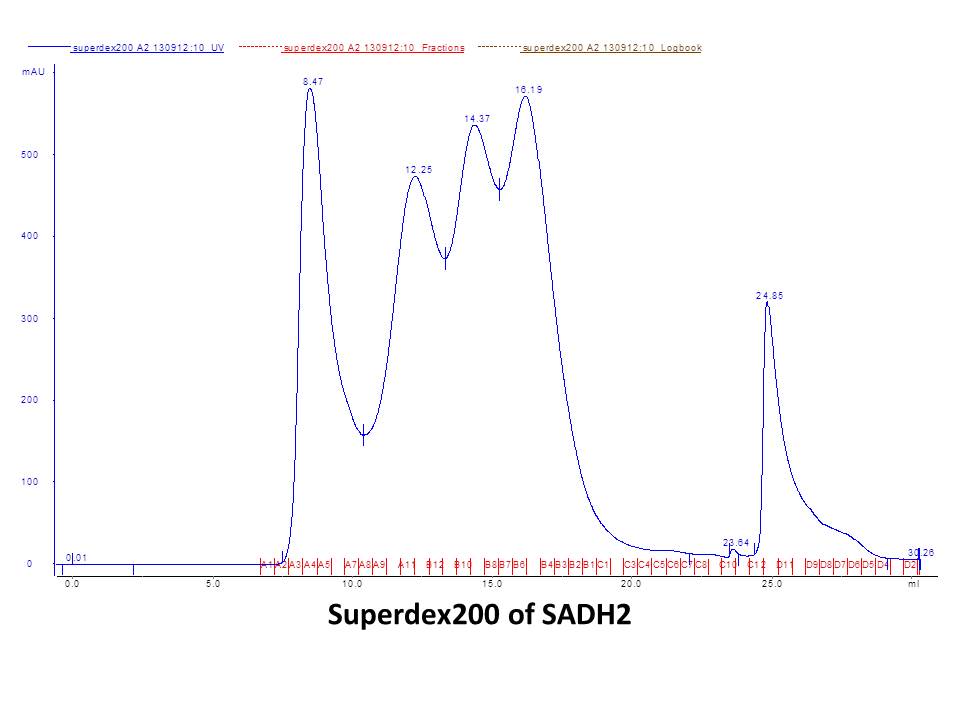
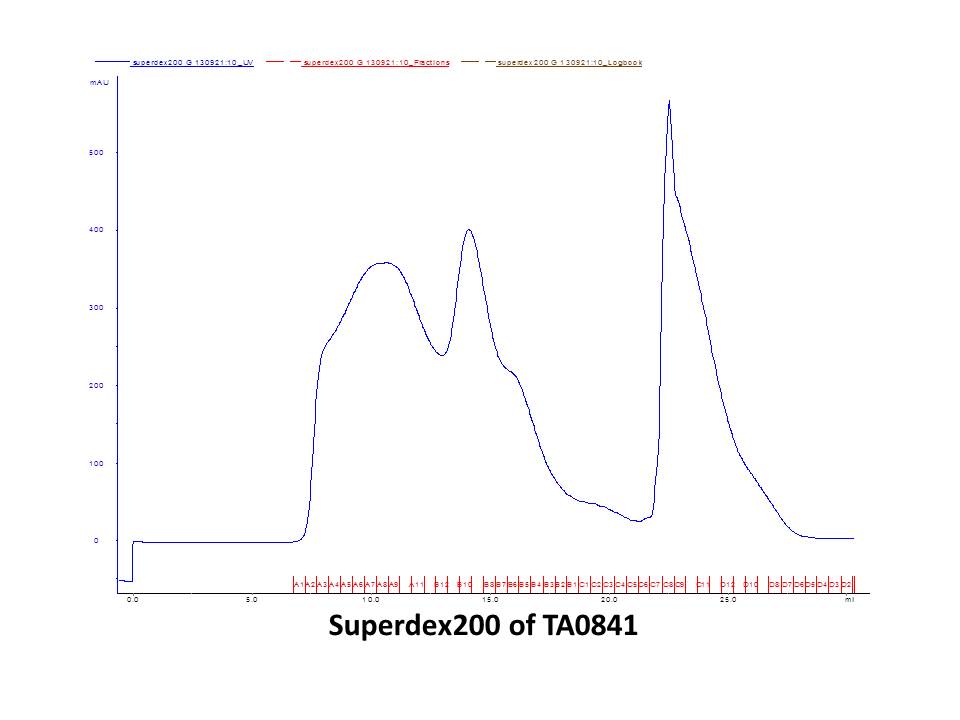
September 5thWet lab DNA sequencing for pET-28a(+)-ta0841 (3rd) and pET-28a(+)-Nadh1 (7st) Result:positive! positive! positive! ~\(≧▽≦)/~ Dry lab September 6thWet lab Digestion PSB1C3 backbone by PstI+EcoRI and gel check Gel purification of digested PSB1C3 Dry lab Design primers for biobricks September 8thLab meeting Discussion for experiment and T-shirt September 9thWet lab Primers for biobricks arrive Digestion of Sadh2with EcoR1 and Pst1 Digestion of Nadh1 with EcoR1 and Pst1 Column-purification of digested gene Nanoview: the concentration is OK O(∩_∩)O~ Ligation of Sadh2 with pET-28a(+) Ligation of Nadh1 with pET-28a(+) Dry lab September 10thWet lab Transformation of ligated PSB1C3- Sadh2 (1st) andPSB1C3-Nadh1 (1st) Result: no colony! ( ⊙o⊙ ) Dry lab September 11thTransformation of ligated PSB1C3- Sadh2 (2st) andPSB1C3-Nadh1 (2st) Result: no colony! o(︶︿︶)o Analysis for the bad result Dry lab September 12thWet lab Digestion of Sadh2 with EcoR1 and Pst1 Digestion of Nadh1 with EcoR1 and Pst1 Column-purification of digested gene Nanoview: the concentration is high O(∩_∩)O~ Ligation of Sadh2 with pET-28a(+) Ligation of Nadh1 with pET-28a(+) Dry lab September 13thWet lab Transformation of ligated PSB1C3- Sadh2 (3rd) and PSB1C3-Nadh1 (3rd) Result: look! The plate of Sadh2 has colony! Yeh~ Small inoculation of PSB1C3-Sadh2 (3rd) Dry lab September 14thWet lab Plasmid extraction of PSB1C3-Sadh2 (3rd) for identification Result:positive results!!! ~\(≧▽≦)/~ Bravo~ Dry lab September 15thLab meeting Presentation rehearsing September 16thWet lab Error-pone PCR of Sadh2, Nadh1 and ta0841 for random mutagenesis Mailing our biobrick Plasmid maximum extraction(alkaline lysis method) Gel electrophoresis of Error-pone PCR product Gel purification of Error-pone PCR product Transformation of pET-28a(+)-Sadh2 into BL21(Rosetta) Dry lab September 17thWet lab Inoculation for pET-28a(+)-Sadh2 Protein induction with 0.6mM IPTG,16℃,over night Dry lab September 18thCell lysis Affinity chromatography with a Nickel column Purification by gel-filtration chromatography using the Superdex 200 High Performance column SDS-PAGE for protein induced assay Result:our protein has been induced! \(^o^)/
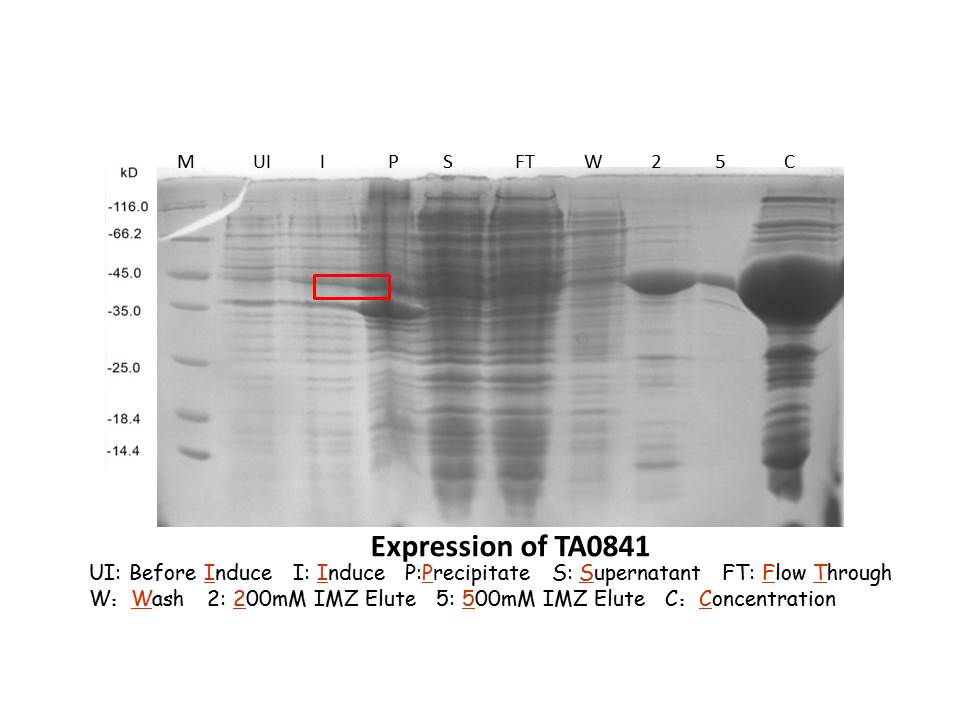
September 19thWet lab Digestion of Error-Pone Sadh2and ta0841with Bam1-HF and Sal1-HF Digestion of Error-Pone Nadh1 with BamH1-HF and Not1-HF Column-purification of digested gene Nanoview: the concentration is high O(∩_∩)O~ Ligation of purification products with pET-28a(+) Transformation of pET-28a(+)-Nadh1 and pET-28a(+)-ta0841 into BL21(Rosetta) Dry lab Mathematical modeling Information about the Judging Forms can be found in our Model September 20thWet lab Transformation of conjunction into BL21 competent cells Inoculation for pET-28a(+)-ta0841 and pET-28a(+)-Nadh1 Protein induction with 0.6mM IPTG,16℃,over night Dry lab September 21stCell lysis Affinity chromatography with a Nickel column Purification by gel-filtration chromatography using the Superdex 200 High Performance column SDS-PAGE for protein induced assay Result: ta0841 has been induced,but Nadh1 isn’t. |
 "
"