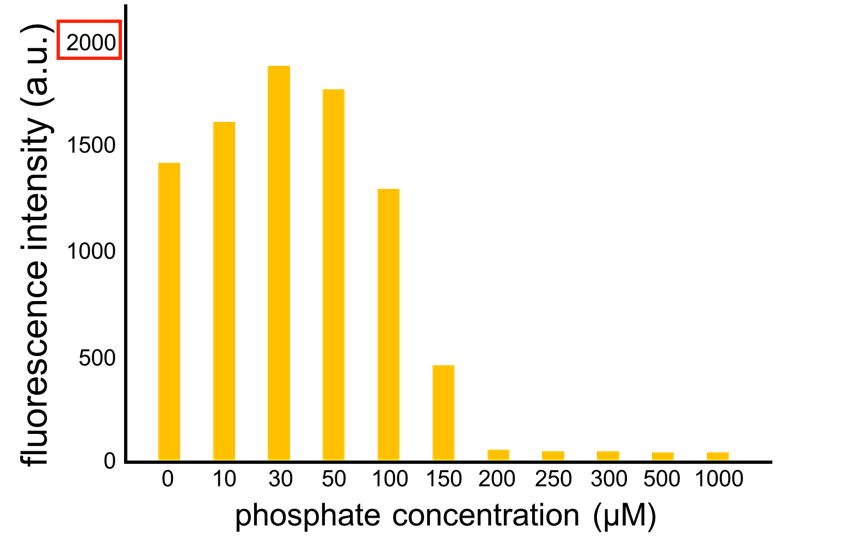Team:Tokyo Tech/Submitted Parts
From 2013.igem.org
Parts submitted to the Registry
For a brief overview of our main results, please have a look at our Main Results page. Go to “Home”
Favorite Tokyo_Tech 2013 iGEM Team Parts
| Name | Type | Description | Designer | Length | |
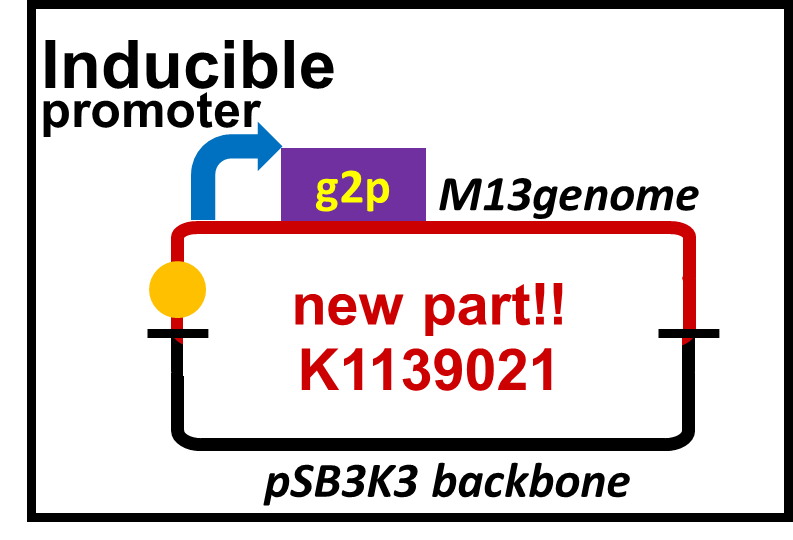
| W | [http://parts.igem.org/Part:BBa_K1139021 BBa_K1139021] | Composite | Plux-M13-Plac-GFP | Naoki Watarai | 7705 |
| W | [http://parts.igem.org/Part:BBa_K1139110 BBa_K1139110] | Composite | Pcon-LasR-Plux/tet-GFP | Naoki Watarai | 1888 |
| W | [http://parts.igem.org/Part:BBa_K1139201 BBa_K1139201] | Measurement | PphoA-GFP-TT | Sara Ogino | 1017 |
Tokyo_Tech 2013 iGEM Team Parts
| Name | Type | Description | Designer | Length | |
| [http://parts.igem.org/Part:BBa_K1139019 BBa_K1139019] | Composite | Promoterless-M13 | Naoki Watarai | 6400 | |
| W | [http://parts.igem.org/Part:BBa_K1139020 BBa_K1139020] | Composite | PlacIQ-M13-Plac-GFP | Naoki Watarai | 7406 |
| [http://parts.igem.org/Part:BBa_K1139150 BBa_K1139150] | Measurement | PRM/lac-GFP-TT | Naoki Watarai | 1032 |
Best new BioBrick part (natural and engineered): [http://parts.igem.org/Part:BBa_K1139021 BBa_K1139021]
M13 is a filamentous phage that infects only F+ strains of E. coli, which does not kill the host cell. This biobrick is extracted from M13mp18 phage vector by PCR. It includes 11 ORFs, M13 origin, a packaging sequence and lac promoter. The promoter on the upstream of g2 (gene 2) is altered to lux promoter. A phage particle is formed only when the host cell receives AHL signal (3OC6HSL, C6) because g2p (gene 2 protein) is an endonuclease needed for a plasmid to be replicated by M13 origin, and to be packaged into the phage particle. As a reporter, GFP is inserted on the downstream of the lac promoter.
Best new BioBrick part (natural): [http://parts.igem.org/Part:BBa_K1139020 BBa_K1139020]
We confirmed that M13 genome with two modifications related to our design kept plaque forming activity. One is replacement of the promoter for G2p protein with a constitutive promoter, PlacIq ([http://parts.igem.org/Part:BBa_I14032 BBa_I14032]). The other is accommodation of pSB3K3 backbone. Even though the plasmid has two different types of replication origins, M13 origin and pSB3 origin, this plasmid ([http://parts.igem.org/Part:BBa_K1139020 BBa_K1139020], Fig. 5-1-2) formed plaque. In contrast, construction intermediates without a promoter for g2p coding sequence (Promoterless-M13 + Plac, Promoterless-M13 + Plac-GFP [http://parts.igem.org/Part:BBa_K1139022 BBa_K1139022], Fig. 5-1-3) could not form plaque.
Best improved part and Best new BioBrick part (natural):
[http://parts.igem.org/Part:BBa_K1139201 BBa_K1139201]
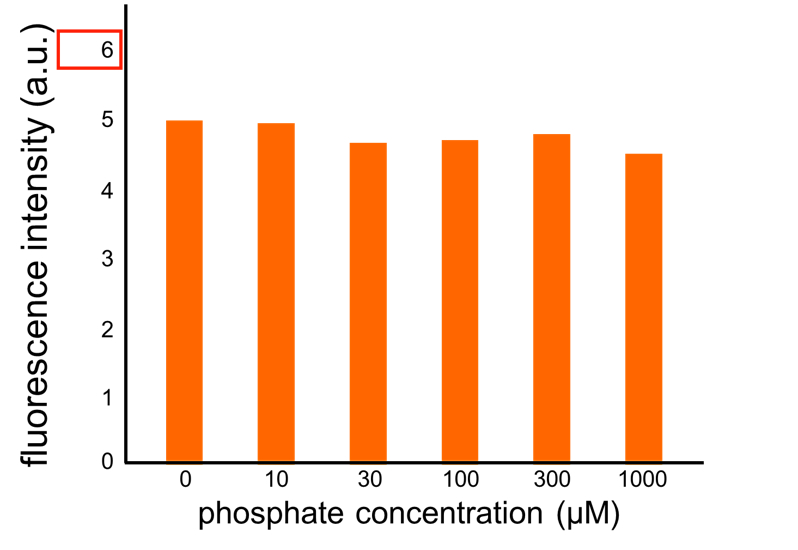
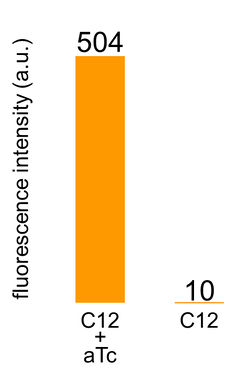
We improved a phosphate sensor part since the existing phosphate sensor part (OUC-China 2012, [http://parts.igem.org/Part:BBa_K737024 BBa_K737024]) did not have sufficient data. We constructed this part by amplifying the phoA promoter region of E. coli (MG1655) and ligating it upstream of GFP part. Compared to OUC-China’s phosphate sensor part including phoB promoter (Fig. 5-1-5), our phosphate sensor part shows clearer result (Fig. 5-1-4).
Best new BioBrick part (engineered): [http://parts.igem.org/Part:BBa_K1139110 BBa_K1139110]
We constructed [http://parts.igem.org/Part:BBa_K1139110 BBa_K1139110] by combining Pcon-lasR ([http://parts.igem.org/Part:BBa_K553003 BBa_K553003]) and Plux/tet-GFP ([http://parts.igem.org/Part:BBa_K934025 BBa_K934025]). This is the first Biobrick part that succeeded in confirming circumvention of crosstalk of LasR to lux promoter. Using this part with plasmid that is constitutively expressing luxR and tetR, we succeeded in confirming circumvention of crosstalk LasR to lux promoter. To know more about crosstalk circumvention assay, please see here.
 "
"