Team:Imperial College/Western Blots
From 2013.igem.org
Western Blots
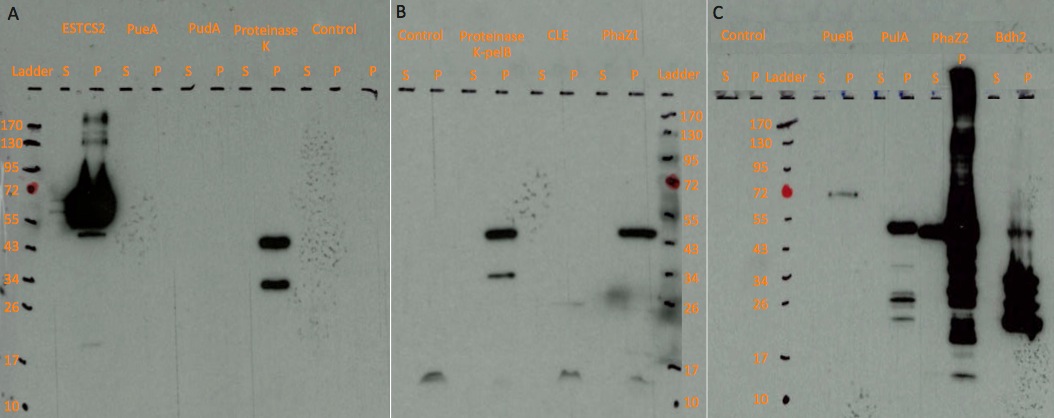
Western blot analysis to determine expression and secretion of our constructs. Expression was induced with either Arabinose or Xylose as appropriate and cell lysate and supernatant samples were collected 5 hours post induction.
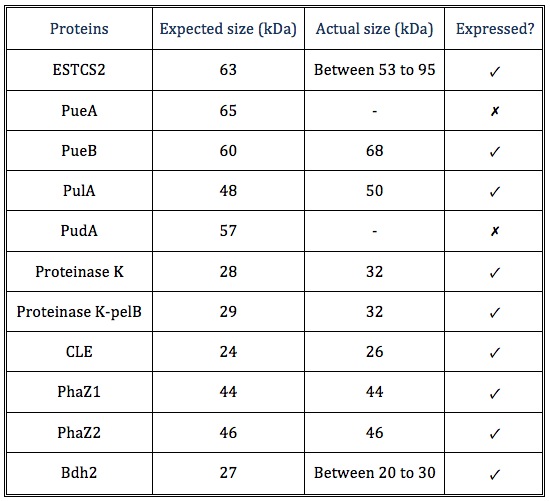
Result summary
The actual sizes of some proteins tend to be larger than the expected sizes, probably because these proteins are expressed differently in E. coli compared to the original host, or the protein may have interacted with molecules in the cell.
Some very strong signals may be the result of high inducer concentration or overloading sample in the wells, thus the concentrated protein samples left signal while moving towards the anode. The small weak bands may be the degraded protein parts.
A dark, larger than expected band is present in both proteinase K and proteinase K-pelB sample. This band might be the degraded protein part interacting with other molecules, because it presents in both proteinase K and proteinase K-pelB samples. They could also have been contaminated by PhaZ1 sample, as the unexpected protein band has a similar size to PhaZ1. The latter less likely, since DNA were tested on gel and no contamination was detected.
We are currently optimising protein secretion using our secretion toolkit.
 "
"