Team:Imperial College/BioPlastic Recycling: PHB
From 2013.igem.org
Module 2: Plastic Fantastic
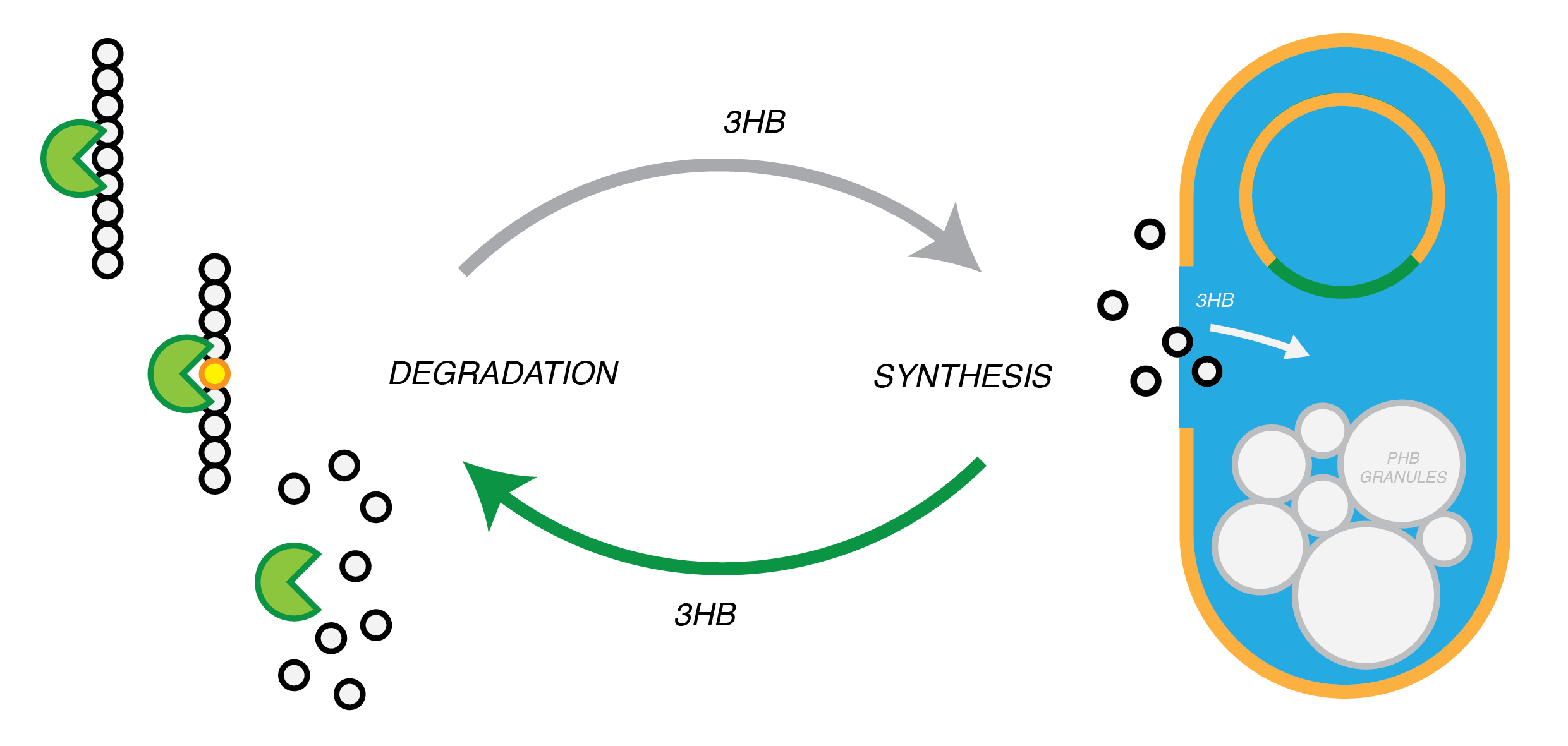
Plastic Fantastic is a complete P(3HB) bioplastic recycling platform, where P(3HB) is degraded into monomeric form and then re-polymerised back into de novo P(3HB) for future applications.
- Overview
- Specifications
- Design
- Modelling
- Assemble
- Testing & results
- Future work
Overview

We are developing a system by which PHB can be recycled when products made from it come to the end of their life. In in order to do this, we have engineered E.coli to break down P3HB extracellularly to release the monomers of 3-hydroxybutyrate (3-HB). E.coli in a different bioreactor will then use the 3-HB as their carbon source for the reproduction of P3HB. We have thoroughly characterised the phaZ1 PHB depolimerase enzyme and demonstrated that it degrades PHB in various experiments. Our models predict that use of the enzyme on a bioreactor scale will be effective in degrading bioplastic on an industrial or local scale. For the adjustment of the PHB synthesis pathway, we designed a metabolic pathway where a permease will take up 3HB and the bdh2 dehydrogenase enzyme will convert it to acetoacetate, which can be then used for PHB synthesis.
We are also establishing a second bioplastic recycling platform for polylactic acid (PLA)
This aspect of our module is in collaboration with the Yale iGEM team
Specification
Degradation of P(3HB)
Our bacteria should be resist any toxic effects that are associated with P(3HB) or 3HB
Our bacteria should degrade (P3HB)
Synthesis of P(3HB)
Our bacteria should take up and internalise 3HB from the surrounding media
Our bacteria should be able to utilise P(3HB) as a sole carbon source
Design
In order to ensure efficient expression in our Chassis, we ordered E.coli codon optimized versions of our proetin coding genes.
Degradation of P(3HB)
We are using E.coli chasis and we made sure to test for relevant toxicity issues, please see our data. PHB and 3HB are not toxic to the cells under conditions relevant to our bioreactor design.
[http://parts.igem.org/Part:BBa_K1149010 BBa_K1149010]: Extracellular expression of phaZ1, PHB depolymerase enzyme. It is regulated by a strong xylose the inducible promoter [http://parts.igem.org/Part:BBa_I741018 BBa_I741018] and we are using the RBS 0034. For extracellular secretion, we fused pelB secretion tag [http://parts.igem.org/Part:BBa_J32015 BBa_J32015] to the N terminus of the protein.
 http://www.igem.org/wiki/images/8/86/Reaction_phaz.jpg
http://www.igem.org/wiki/images/8/86/Reaction_phaz.jpg
Synthesis of P(3HB) from 3HB
3HB Permease:We designed a biobrick for the expression of a Putative permease identified from the literature [http://www.ncbi.nlm.nih.gov/nuccore/AB330992(AB330992.1)] for 3HB inport. We optimised the sequence for E.coli and planned experiments with the construct. However, the gene synthesis was delayed and we did not get to the stage of characterizing and submitting the part.
[http://parts.igem.org/Part:BBa_K1149050 BBa_K1149050]: Intracellular expression of bdh2, 3HB dehydrogenase enzyme . We have used a strong arabinose inducible promoter [http://parts.igem.org/Part:BBa_K206000 BBa_K206000] in front of the operon for controlled expression of the enzyme since the enzyme is only necessairy for the cell if 3HB is present. We have included superfolder GFP [http://parts.igem.org/Part:BBa_K515005 BBa_K515005] in operon with phaZ1 so that we can monitor gene expression from the promoter via fluorescence measurements. (see our corresponding data under results)

http://www.igem.org/wiki/images/f/f0/Reaction_bdh.jpg
Safety
Bacteria strains
We used E. coli K-12 strains MG1655, NEB 10 beta, NEB 5 alpha and TOP10 (similar to E. coli K-12 DH 10 beta that fall under Risk Group 1 for the experiments, but eventually only MG1655 strain will be used in the bioreactors. This strain is shown rarely survives outside labs, although minor symptoms may show if infected. Meanwhile, the parts we designed are specialised for degrading PUR and they are not expected to harm human health.
Chemical reagents
Some of the reagents we used were slightly toxic to human. In order to reduce the risks of using toxic reagents, we always wear gloves and labcoats. For some toxic reagents, we performed in fume cupboard. Click on the reagents to see the MSDS.
1. [http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=GB&language=en&productNumber=363502&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F363502%3Flang%3Den Poly(R)-3-hydroxybutyric acid].
2. [http://www.sigmaaldrich.com/MSDS/MSDS/PleaseWaitMSDSPage.do?language=&country=GB&brand=SIAL&productNumber=P2308&PageToGoToURL=http://www.sigmaaldrich.com/catalog/product/sial/p2308?lang=en®ion=GB Proteinase K].
3. [http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=GB&language=en&productNumber=54965&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F54965%3Flang%3Den Sodium 3-hydroxybutyrate].
4. [http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=GB&language=en&productNumber=A3256&brand=SIGMA&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Fsigma%2Fa3256%3Flang%3Den Arabinose].
5. [http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=GB&language=en&productNumber=A8509&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2Fa8509%3Flang%3Den Acetoacetate].
Bioreactor safety
We have come up with a few strategies to prevent release of our bacteria into the environment. Firstly, the bioreactor containing bacteria will have a kill switch that kills bacteria if the bioreactor is opened when operating. Secondly, we lyse the cells at the end of reactions to harvest our product. This also ensures living bacteria will not be released unexpectedly.
Enzyme activity of PHB depolymerase (phaz1)
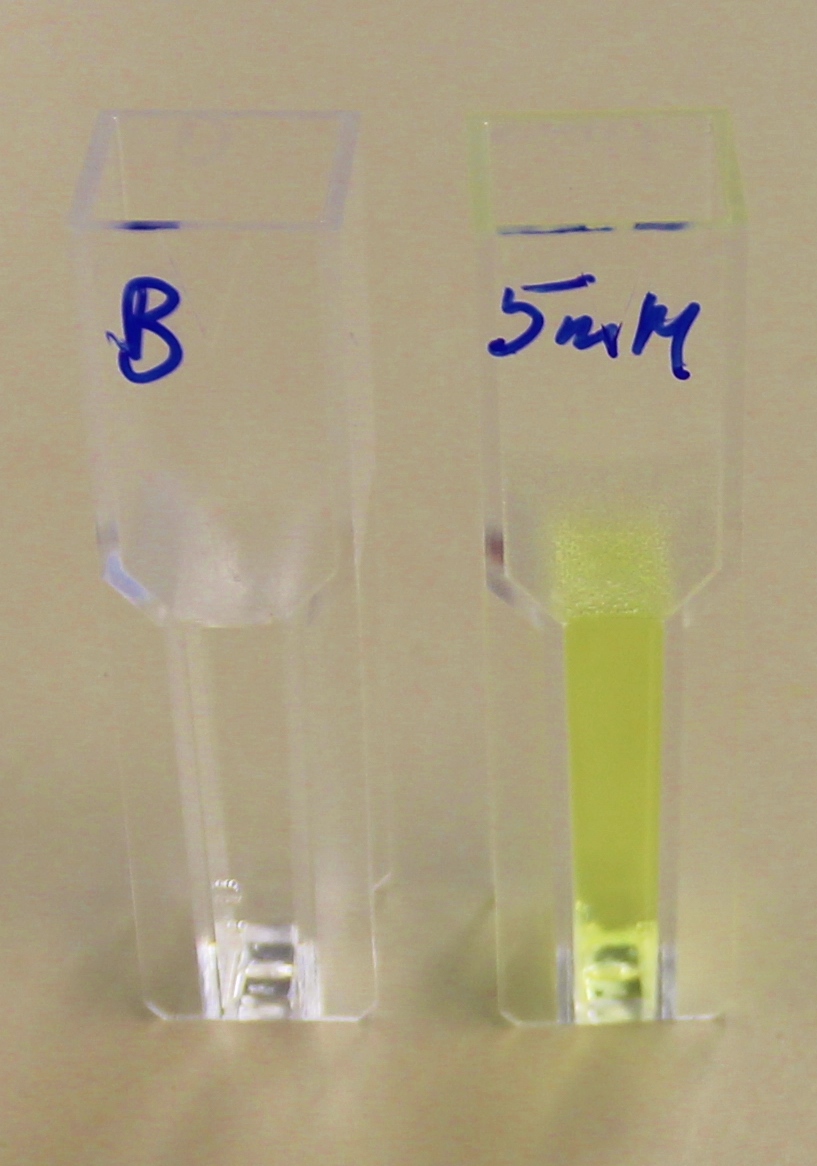
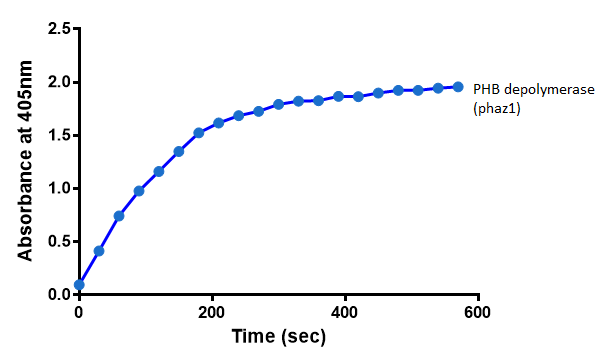
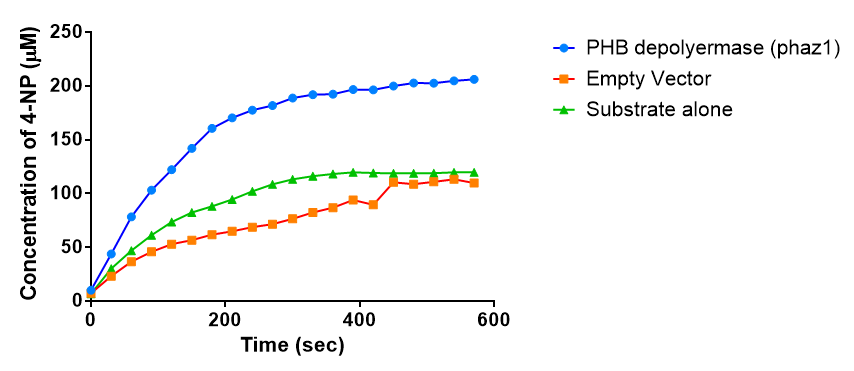
It can be seen from the Western Blot results that the PHB depolymerase (phaz1) [http://parts.igem.org/Part:BBa_K1149010 BBa_K1149010] was being expressed. To show that this enzyme has esterase activity, colourimetric assays were performed using the substrate analog para-Nitrophenyl butyrate. When the ester bond in this substrate is cleaved, 4-Nitrophenol is released. This is accompanied by an increase in absorbance at the wavelength 405 nm and a colour change from colourless to yellow. The concentration of 4-Nitrophenol produced could then be calculated with the Beer-Lambert Law, as the extinction coefficient of 4-NP at 405 nm is 18,000 M-1 cm-1. This experiment was performed with both the crude cell lysate and purified PHB depolymerase. Our data shows that this enzyme is definitely active.
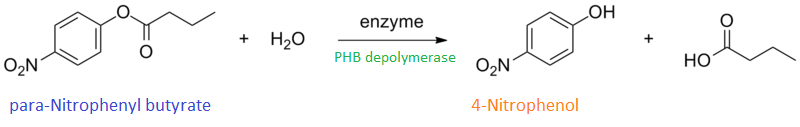
Cell lysate assay
After 48 hours of growing and inducing the phaz1 culture, the cells were lysed by probe-sonication, spun down and resuspended in 50mM Tris-HCl buffer. A reaction mixture containing 5 µL of crude phaz1 lysate, 4 µL of para-Nitrophenyl butyrate and 1 mL of 50 mM Tris-HCl pH 7.4 buffer was incubated in the [http://www.eppendorf.com/int/index.php?sitemap=2.1&action=products&contentid=1&catalognode=87236 Eppendorf BioSpectrometer] for 570 seconds, whilst the absorbance at 405 nm was automatically recorded every 30 seconds.
From the above graph, it can be seen that greater esterase activity occurs when the phaz1 cell lysate is in the reaction mixture. The graph shows that there is also esterase activity occuring in the Empty Vector and Substrate alone reaction mixtures, but this is due to the imidazole present in the Tris-HCl buffer, which acts as a general base catalysis.[http://pubs.acs.org/doi/pdf/10.1021/ja00874a035 (1)]
Results
Purified enzyme assay
Since the phaz1 [http://parts.igem.org/Part:BBa_K1149010 BBa_K1149010] construct contains a His tag, the protein could be purified by metal affinity chromatography.
- Thomas C. Bruice , Thomas H. Fife , John J. Bruno , Patricia. BenkovicHydroxyl Group (V)1 and Imidazole (X)2 Catalysis. The General Base Catalysis of Ester Hydrolysis by Imidazole and the Influence of a Neighboring Hydroxyl Group. J. Am. Chem. Soc., 1962, 84 (15), pp 3012–3018
- ANDERSON A, DAWES E. Occurrence, Metabolism, Metabolic Role, and Industrial Uses of Bacterial Polyhydroxyalkanoates. Microbiol Rev 1990 DEC;54(4):450-472.
- Harding KG, Dennis JS, von Blottnitz H, Harrison STL. Environmental analysis of plastic production processes: Comparing petroleum-based polypropylene and polyethylene with biologically-based poly-beta-hydroxybutyric acid using life cycle analysis. J Biotechnol 2007 MAY 31;130(1):57-66.
- Kim S, Dale BE. Energy and Greenhouse Gas Profiles of Polyhydroxybutyrates Derived from Corn Grain: A Life Cycle Perspective. Environ Sci Technol 2008 OCT 15;42(20):7690-7695.
- Jendrossek D, Handrick R. Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 2002;56:403-432.
- Philip S, Keshavarz T, Roy I. Polyhydroxyalkanoates: biodegradable polymers with a range of applications. Journal of Chemical Technology and Biotechnology 2007 MAR;82(3):233-247.
Proteinase K is a hydrolytic enzyme and active
Proteinase K is expressed in MG1655 cells transformed with BBa_K1149008
Proteinase K degrades PLA cup
Below come all the fabulous SEM images we shall hopefully have: https://static.igem.org/mediawiki/parts/8/83/PLA_SEM_plans.jpgbdh2 improves growth on 3HB
We have previously observed that MG1655 cells can survive in minimal media that contains 3HB as sole carbon source. This is evidence for that some uptake mechanism and a metabolic pathway is active at a low level in the cells. We hypothesised that the 3HB dehydrogenase could improve growth since it can convert 3HB into acetoacetate, a common metabolite in E.coli.
In order to test this, we run a growth experiment with various 3HB concentrations where the growth of cells containing bdh2 (BBa_K1149050) and Empty vector was recorded on a 96 well plate. We have calculated the growth rates from this data and plot it on the graph below.
https://static.igem.org/mediawiki/2013/0/0d/M9S_%283HB%29.pngWe can conclude that there is a significant increase in growth rate of bdh2 containing cells at 10 000 uM 3HB. (stats!) This suggests that bdh2 is able to function as expected and produces acetoacetate which is used by the cell`s central metabolic pathways for growth.
At lower 100 or at higher 100 000 uM, we did not observe difference in growth. This could be be because the rate limiting step at low concentrations could be the uptake of 3HB rather than it`s conversion to acetoacetate. We hope that we could observe an increase in growth if we added the putative permease we designed to the system. At higher level, there is a drop in growth rates in both bdh2 and control cells, probably because of toxicity issues.
Safety
Our project used several potentially harmful chemicals. Ensure you know the risks involved with chemicals you use by checking the full material safety data sheet(MSDS)
 "
"