Team:Paris Bettencourt/Project/Infiltrate
From 2013.igem.org

Background:
Latent tuberculosis persists inside macrophages of the lungs, where it is partially protected from both the host immune system and conventional antibiotics.
Aim:
To create an E. coli strain capable of entering the macrophage cytosol and delivering a lytic enzyme to kill mycobacteria.
Results:
- We expressed the enzyme Trehalose Dimycolate Hydrolase (TDMH) in E.coli and showed that it is highly toxic to mycobacteria in culture.
- We expressed the lysteriolyin O (LLO) gene in E. coli and showed that it is capable of entering the macrophage cytosol.
- We co-infected macrophages with both mycobacteria and our engineered E. coli to characterize the resulting phagocytosis and killing.
BioBricks:
-BBa_K1137008 (TDMH)
Introduction
Mycobacterium tuberculosis (Mtb), the bacterium responsible for tuberculosis (TB), spreads by aerosol and infects its host through the airways. The bacterium is phagocytosed by macrophages in the lung, yet often evades death in the lysosome. Mtb can persist for years or even decades inside macrophages by inhibiting phagosome/lysosome fusion and supressing the normal acidification of the lysosome.
An efficient treatment for persistent TB must enter infected macrophages and kill the pathogen there. In our system, E. coli is both the vector and the therapeutic agent agent by expressing the gene LLO to enter macrophages and TDMH to kill mycobacteria.
TDMH and the mycobacterial cell wall
Mycobacterium species share a characteristic cell wall: thick, waxy, hydrophobic, and rich in mycolic acids. The low permeability of the envelope to hydrophilic solutes contributes to the intrinsic drug tolerance in mycobacteria.
Trehalose Dimycolate Hydrolase (TDMH) is a cutinase-like serine esterase that triggers rapid lysis of the mycobacterial cell wall by degrading the mycolate layer. The enzyme was first isolated from Mycobacterium smegmatis and subsequently shown to hydrolyze purified TDM from various mycobacterial species. Exposure to TDMH triggers an immediate release of free mycolic acids, ultimately leading to lysis of many mycobacteria including Mtb (Yang et al. 2012).
We have used M. smegmatis as a model system because Mtb are highly pathogenic and difficult to culture in the lab. M. smegmatis is a close relative of Mtb, shares many of its membrane properties, and is commonly used as a stand-in for Mtb physiology in the lab.
In our system, described in the methods below, we used E. coli BL21 (DE3) as a chassis to express TDMH from an IPTG-inducible strong T7 promoter.
Killing mycobacteria with TDMH
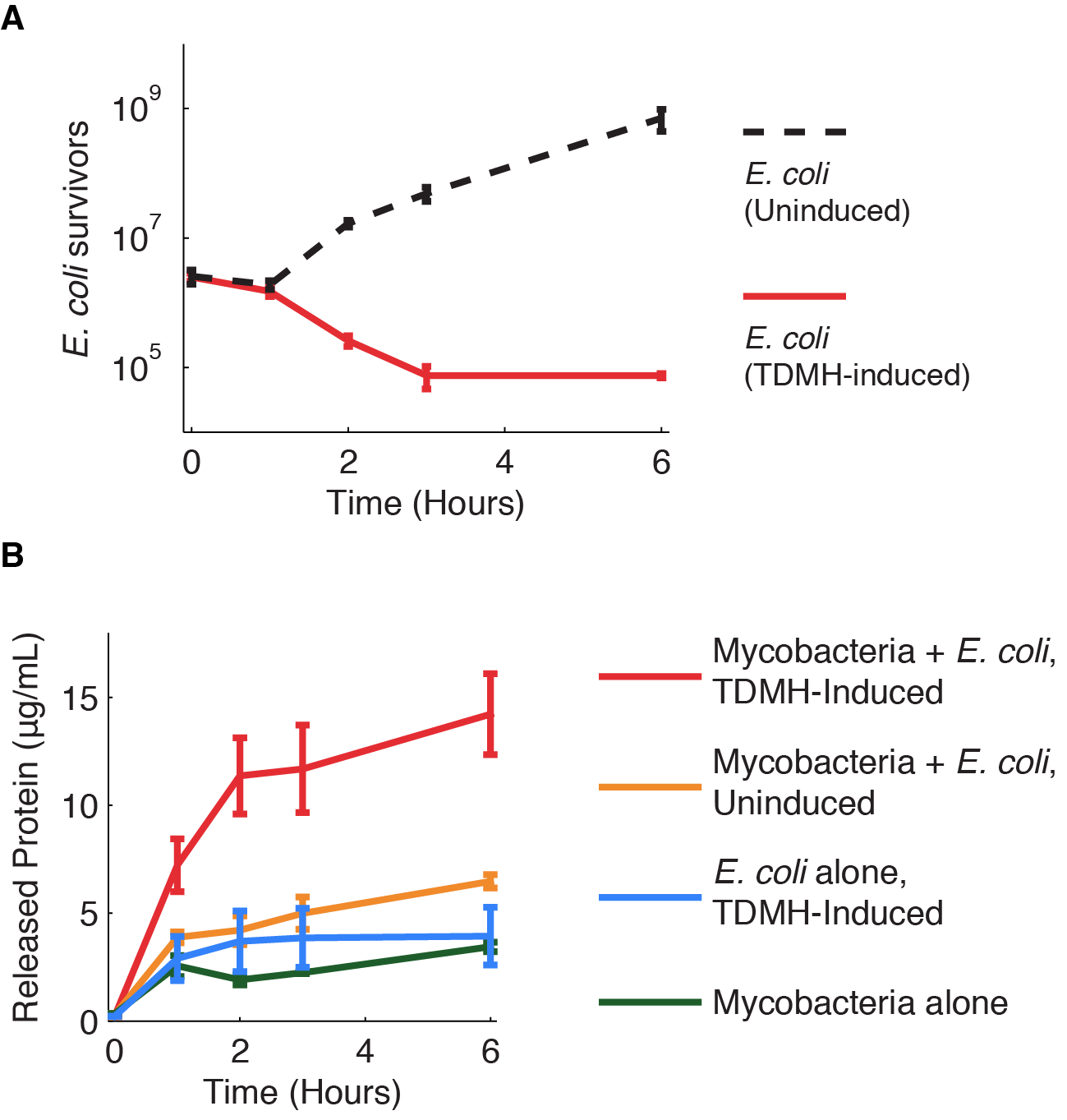
Figure 1 shows the killing of M. smegmatis with TDMH expressed by E. coli. We mixed liquid cultures of E. coli and M. smegmatis at equal cell densities as determined by plating assays. When expression of TMDH was induced with IPTG, nearly 99% of the M. smegmatis were killed within six hours. We saw no change in viability in cultures of M. smegmatis alone or when mixed with uninduced E. coli.
We next sought to quantify the effectiveness of TDMH killing. We mixed induced E. coli and mycobacteria in different ratios and used plating assays to measure viability. As shown in figure 2 mycobacterial killing displayed dose dependence on the E. coli cell density. Small numbers of E. coli could kill many mycobacteria. For example, in mixed populations with 100 mycobactera for each E. coli, we still observed >50% mycobacterial killing after 2 hours. This indicates that, on average, each E. coli produced enough TDMH to kill 50 mycobacterial. We reason that this killing may be even more effective inside macrophages, where constrained volumes will increase the effective TDMH concentration.
TDMH Induction causes lysis and protein release
The TDMH enzyme in our system does not carry a secretion tag. Therefore, lysis of the E. coli membrane is probably required for the protein to reach (and lyse) M. smegmatis. We used plating assays to investigate the effect of TDMH induction on E. coli viability and Bradford assays to measure released protein. The results are presented in figure 2.Figure 2A shows that inducing TDMH-expressing E. coli with IPTG rapidly kills the E. coli. In figure 2B, we show that inducing TDMH expression increases the concentration of free protein in the media. In our model, TDMH induction leads to spontaneous lysis of TDMH-expressing E. coli. This could be simply due to the extremely high protein expression levels driven by the T7 promoter, or it may be partially due to the esterase activities of the TDMH enzyme. Once the TDMH is released from the E. coli it is free to act on mycobacterial membranes, causing lysis and the release of additional proteins.
Bibliography
1. Yang Y, Bhatti A, Ke D, Gonzalez-Juarrero M, Lenaerts A, Kremer L, Guerardel Y, Zhang P, Ojha AK (2012) : Exposure to a cutinase-like serine esterase triggers rapid lysis of multiple mycobacterial species. J Biol Chem. 2013 Jan 4;288(1):382-92.
2. Rajesh Jayachandran, Varadharajan Sundaramurthy, Benoit Combaluzier , Philipp Mueller, Hannelie Korf, Kris Huygen, Toru Miyazaki, Imke Albrecht, Jan Massner, Jean Pieters (2007) : Survival of Mycobacteria in Macrophages Is Mediated by Coronin 1-Dependent Activation of Calcineurin. Cell, Volume 130, Issue 1, 13 July 2007, Pages 12-14



 "
"







 +33 1 44 41 25 22/25
+33 1 44 41 25 22/25


