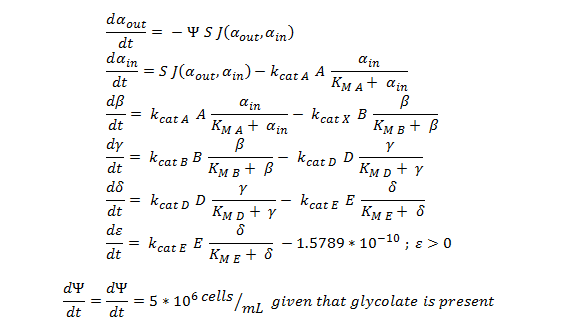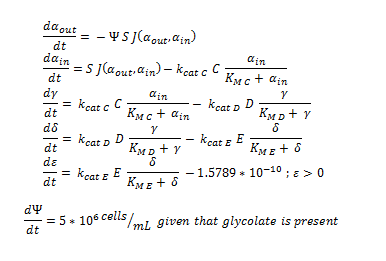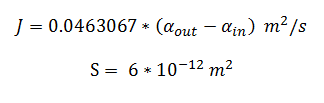Team:SydneyUni Australia/Modelling Model
From 2013.igem.org

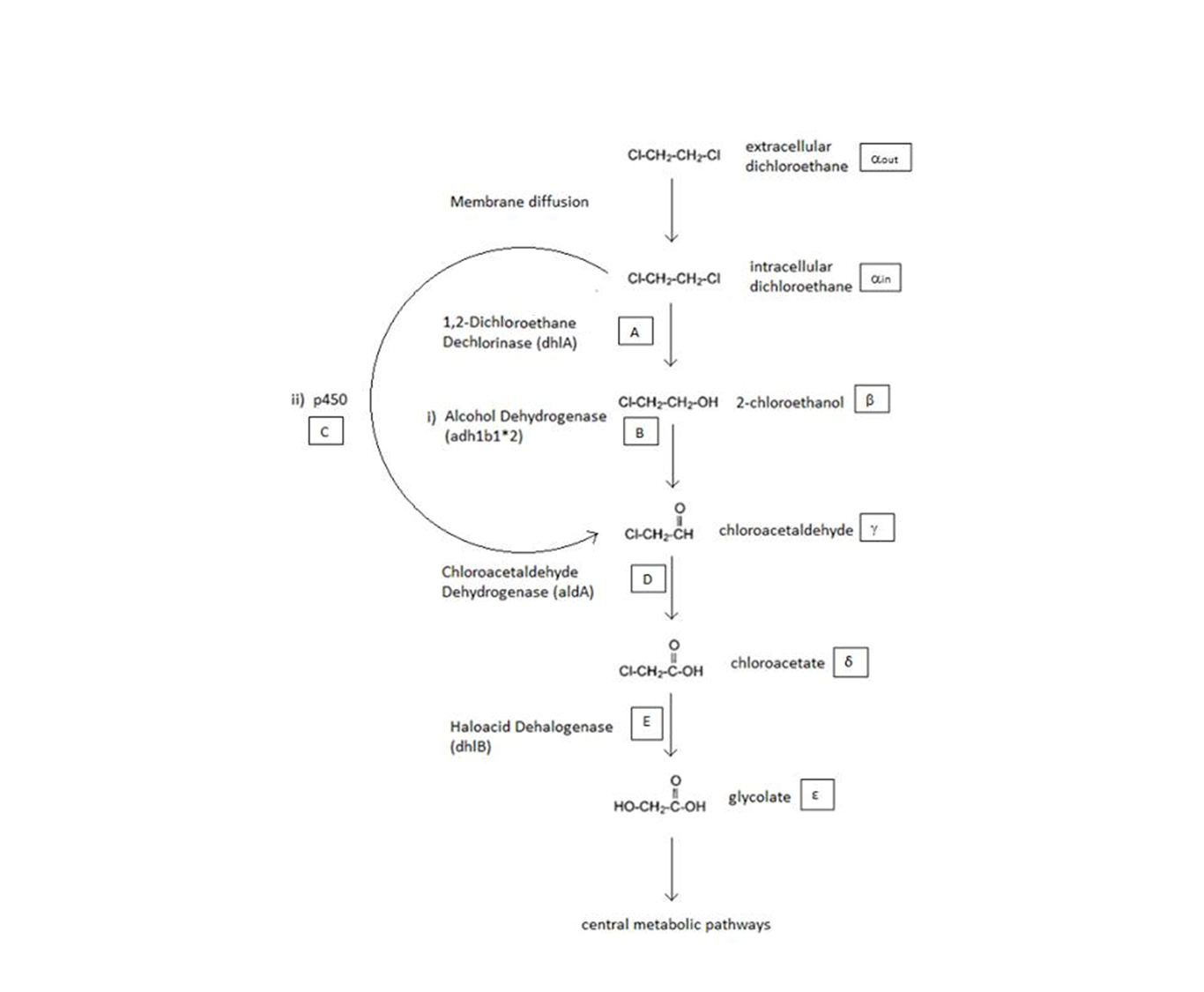
A Schematic of the Engineered Metabolic Pathway:
General Information regarding the Enzymes Involved in the Metabolic Pathway
| Enzyme | Gene | Symbol | Constants | Substrate | Product | Ref |
|---|---|---|---|---|---|---|
| 1,2-Dichloroethane Dechlorinase | dhlA | A | KM A = 0.53 mM, kcat A = 3.3 s-1 | DCA | 2-chloroethanol | [1] |
| Alcohol Dehydrogenase | Adhlb 1/2* | B | KM B = 0.94 mM, kcat B = 0.0871 s-1 | 2-chloroethanol | chloroacetaldehyde | [2] |
| p450 | p450 | C | KM C = 0.120 mM, kcat C = 0.0113 s-1 | DCA | chloroacetaldehyde | [3] |
| Chloroacetaldehyde Dehydrogenase | aldA | D | KM D = 0.06mM, kcat D = 0.60 s-1 | chloroacetaldehyde | chloroacetate | [4] |
| Haloacetate Dehydrogenase | dhlB | E | KM E = 20 mM, kcat E = 25.4 s-1 | chloroacetate | glycolate | [5] |
A table that summarises the enzymes that we used in both of the engineered metabolic pathways. The constants presented were (painstakingly) obtained from the literature (referenced).
ODE Model
Shown below are the two systems of ODEs we used to model each pathway.
The non-monooxygenase pathway (with dhlA (A) and adh1b1*2 (B)).
The monooxygenase pathway (with p450 (C)).
Generally, each line represents how the metabolic intermediate changes over time; this is dictated by the relative rate at which the intermediate is formed vs. the rate at which it is used/removed.
The two above systems of ODEs were used to model two things:
1. The rate at which extracellular DCA is removed from solution (given the number of cells in solution) . This is considering the rate at which DCA crosses the plasma membrane (from solution to cytoplasm) of all cells in solution.
2. The rate at which the intracellular concentrations of the metabolic intermediates change over time within a single cell. This considers the relative activities of the enzymes of the metabolic pathway.
The symbols A, B, C, D & E represent the intracellular enzyme concentrations of 1,2-Dichloroethane Dechlorinase, Alcohol Dehydrogenase, Cytochrome p450, Chloroacetaldehyde Dehydrogenase and Haloacetate Dehydrogenase respectively. They have units of mM.
The symbols αin, β, γ, δ and ε represent the intracellular concentrations of the metabolic intermediates DCA, 2-chloroethanol, chloroacetaldehyde, chloroacetate and glycolate respectively. They have units mM.
αout represents the extracellular concentration of DCA, i.e. the concentration of DCA in solution. It has units mM.
The symbol Ψ represents the concentration of cells in solution and has units cells/mL.
The function J(αout,αin) represents the rate which extracellular DCA moves across the plasma membrane and into the single cell (the flux rate). It is a function of the extra and intracellular concentrations of DCA and has a value of surface area per time m2s-1. The value S represents the average surface area of the cellular membrane of E. coli. By multiplying S by J one achieves the total amount of DCA flowing into a single cell per unit time. The total amount of DCA flowing from solution is achieved by multiplying Ψ by S and J.
Running the Model
The model was run using MATLAB through the ode solver ODE45.
The modelling for both the pathays had initial conditions of:
| Constant | Value | Comment |
|---|---|---|
| KM A | 0.530 mM | From literature [1] |
| kcat A | 3.3 s-1 | From literature [1] |
| KM B | 0.940 mM | From literature [2] |
| kcat B | 0.0871 s-1 | From literature [2] |
| KM C | 7.2 mM | From literature [3] |
| kcat c | 89.8 s-1 | From literature [3] |
| KM D | 0.160 mM | From literature [4] |
| kcat D | 0.600 s-1 | From literature [4] |
| KM E | 20 mM | From literature [5] |
| kcat E | 25.4 s-1 | From literature [5] |
| β, γ, δ & ε | 0 mM | Not naturally present in cells. |
| αout | 1 mM | Initial concentration of DCA in solution (arbitrary) |
| αin | 0.001 mM | Initial concentration of DCA in cell |
| A, B, C, D, E | 25.55 mM | Estimation from literature [11], as described in "principles". |
| 2 x 108 cells / mL | Initial cell concentration which allows appropriate growth |
Physical conditions: the model assumes that the cells are present in minimal media prior to DCA exposure and that the DCA is instantaneously and evenly present at time = 0 min in a solution of homogeneously mixed cells
Many assumptions have been made in the construction of the model and are outlined in the 'principles' section.
By using the constants summarised in the previous section the flux, J, took the value (alongside the bacterial surface area, S):
The Non-monooxygenase Pathway
ODE overview:
The summary of the ODE (explained and justified in the previous section).
Raw MATLAB code:
function dy = nop450(t,y)
dy=zeros(7,1);
dy(1)= -y(7)*(6*10^(-12))*0.0463067*(y(1)-y(2));
dy(2)= ((6*10^(-12))*0.0463067*(y(1)-y(2)))-3.3*25.55*(y(2)/(0.53+y(2)));
dy(3)= 3.3*25.55*(y(2)/(0.53+y(2)))-0.0871*25.55*(y(3)/(0.94+y(3)));
dy(4)= .0871*25.55*(y(3)/(0.94+y(3)))- 0.6*25.55*(y(4)/(0.16+y(4)));
dy(5)= 0.6*25.55*(y(4)/(0.16+y(4))) - 25.4*25.55*(y(5)/(20+y(5)));
if y(6) > 2*10^(-10)
dy(6)= 25.4*25.55*(y(5)/(20+y(5))) -1.5789*10^(-10)
else
dy(6) = 25.4*25.55*(y(5)/(20+y(5)))
end
if y(6) > 0.0005
if y(7) > 1.6*10^11
dy(7)=0
else
dy(7) = 5*10^6
end
else
dy(7) = 0
end
end
MATLAB output:
The rate at which DCA is removed in solution:
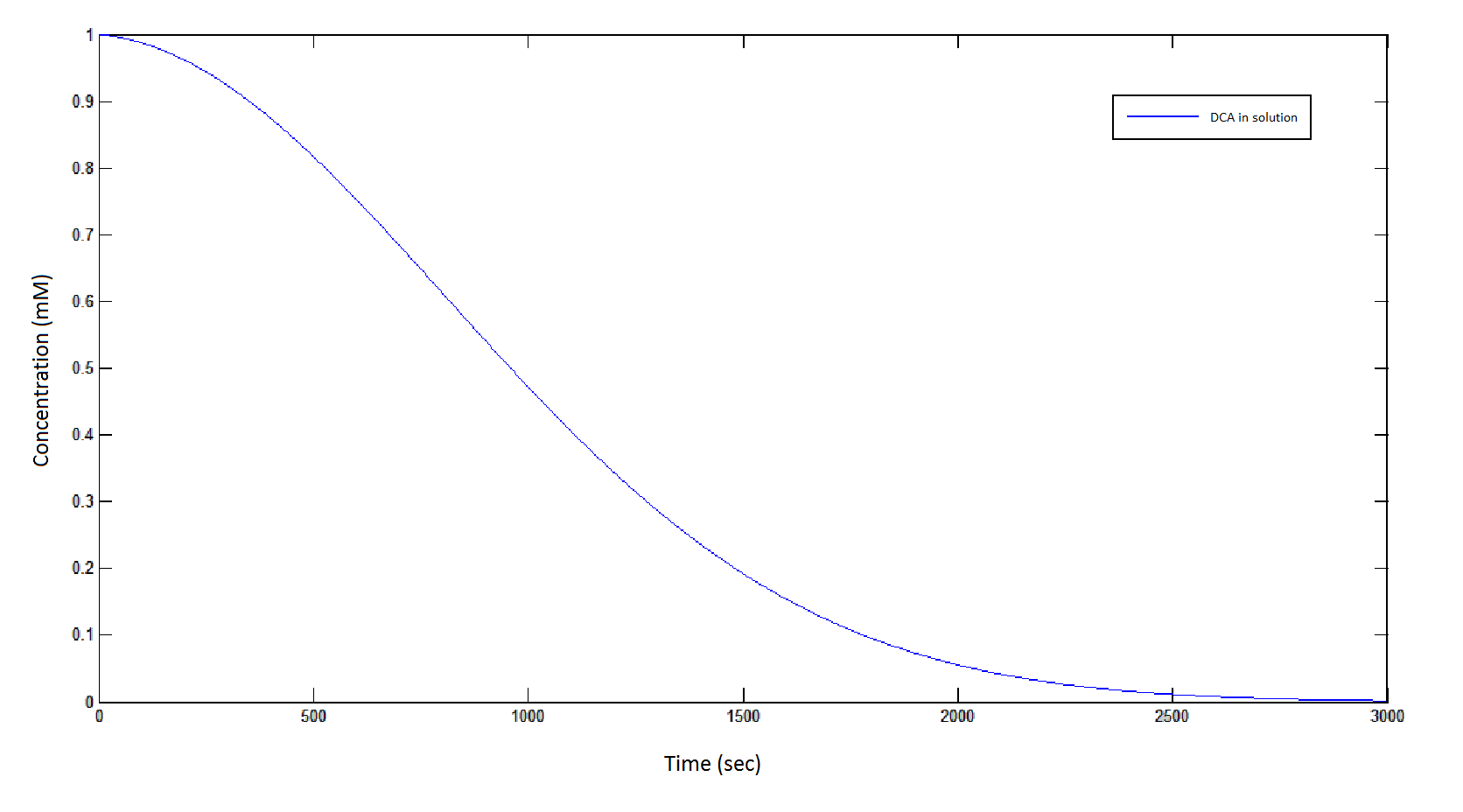
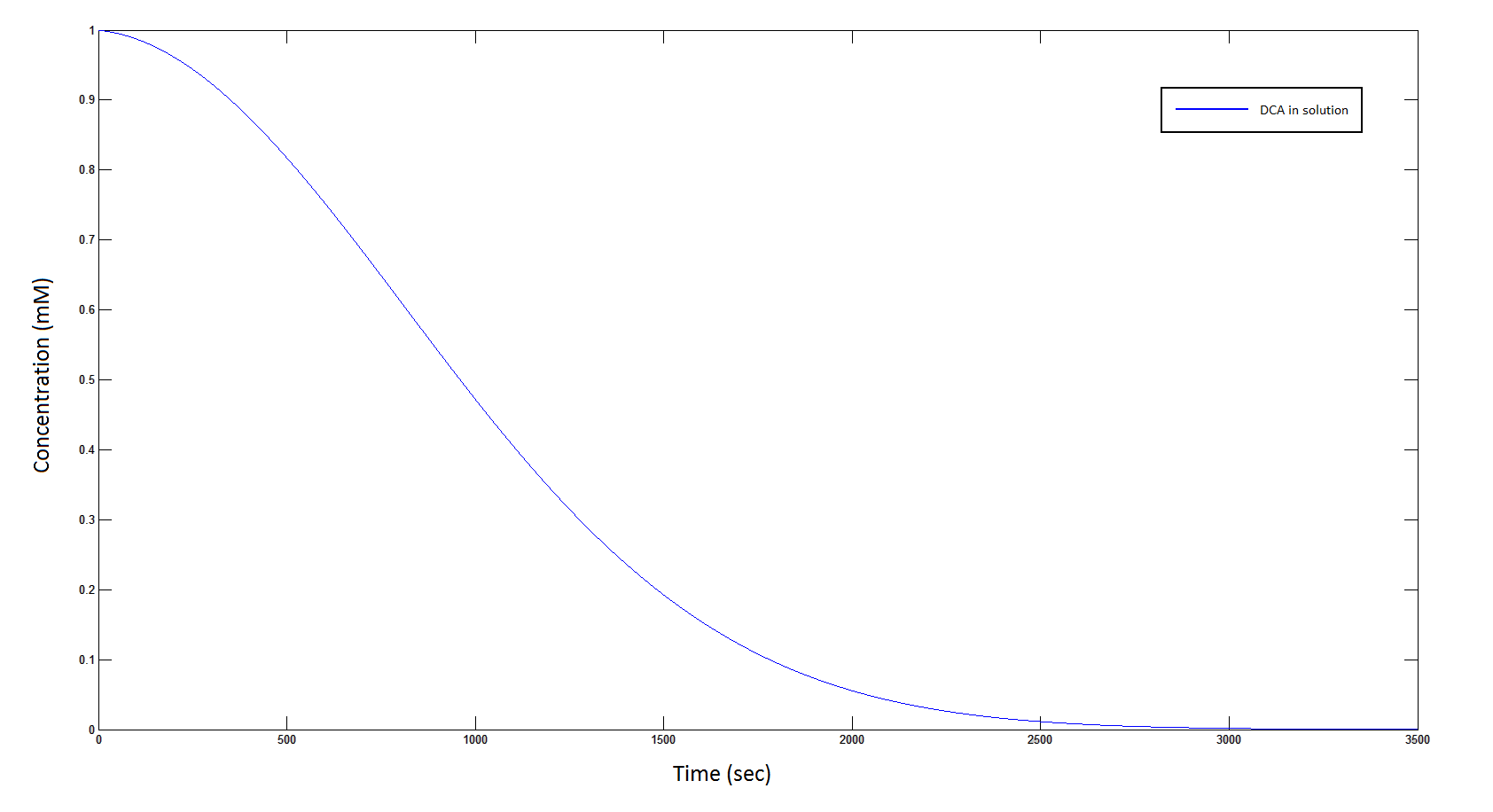
Graph 1: The blue line represents how the concentration of DCA in solution (extracellular) decreases due to the action of our DCA degraders. The red line represents the intracellular concentions of the metabolites (disregard in this graph). One can see that an initial concentration of 2E8 cells/mL completely removes the DCA with a concentration 1mM in (roughly) 150mins.
The Rate at which the intracellular concentration of the metabolites change over time:
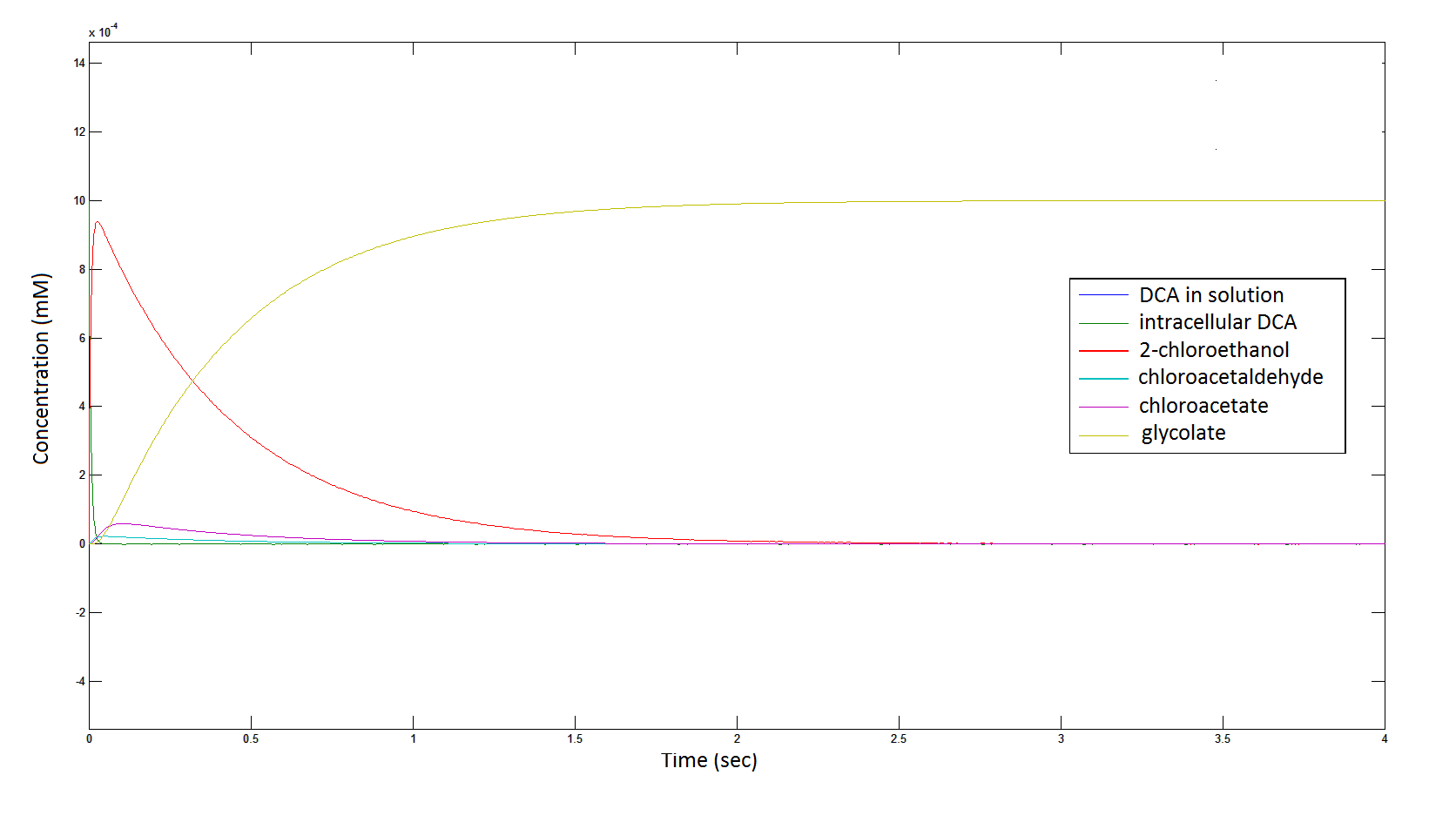
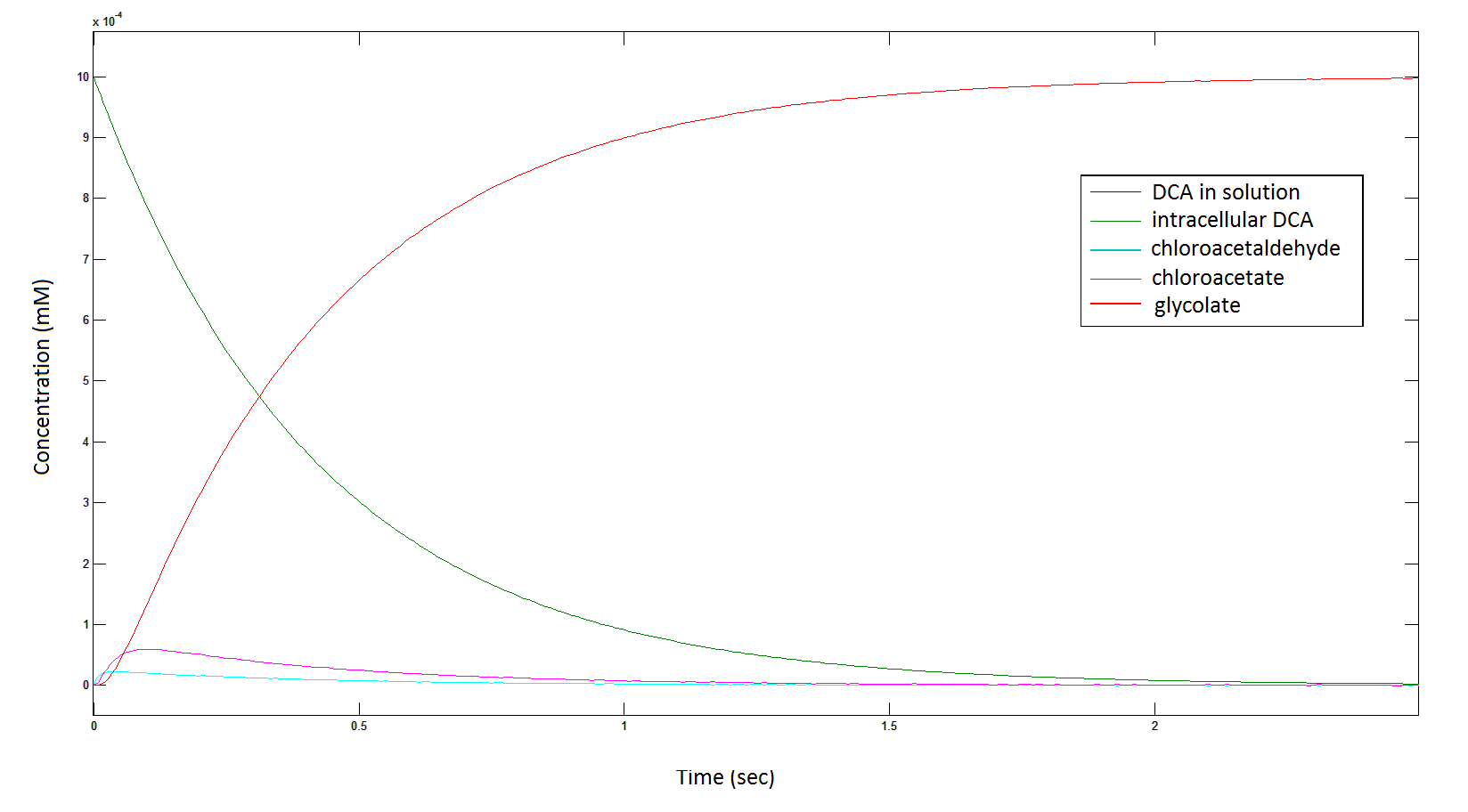
Graph 2: Each line represents the concentration of each of the metabolites . This graph is simply a rescaling of graph 1. Note: the glycolate won’t accumulate in the cell – it is metabolised – the model had glycolate as the final product. It is used to show that the presence of glycolate can attribute to cell growth.
Rescaling the graph once again: the rate at which the metabolic intermediate change over time.
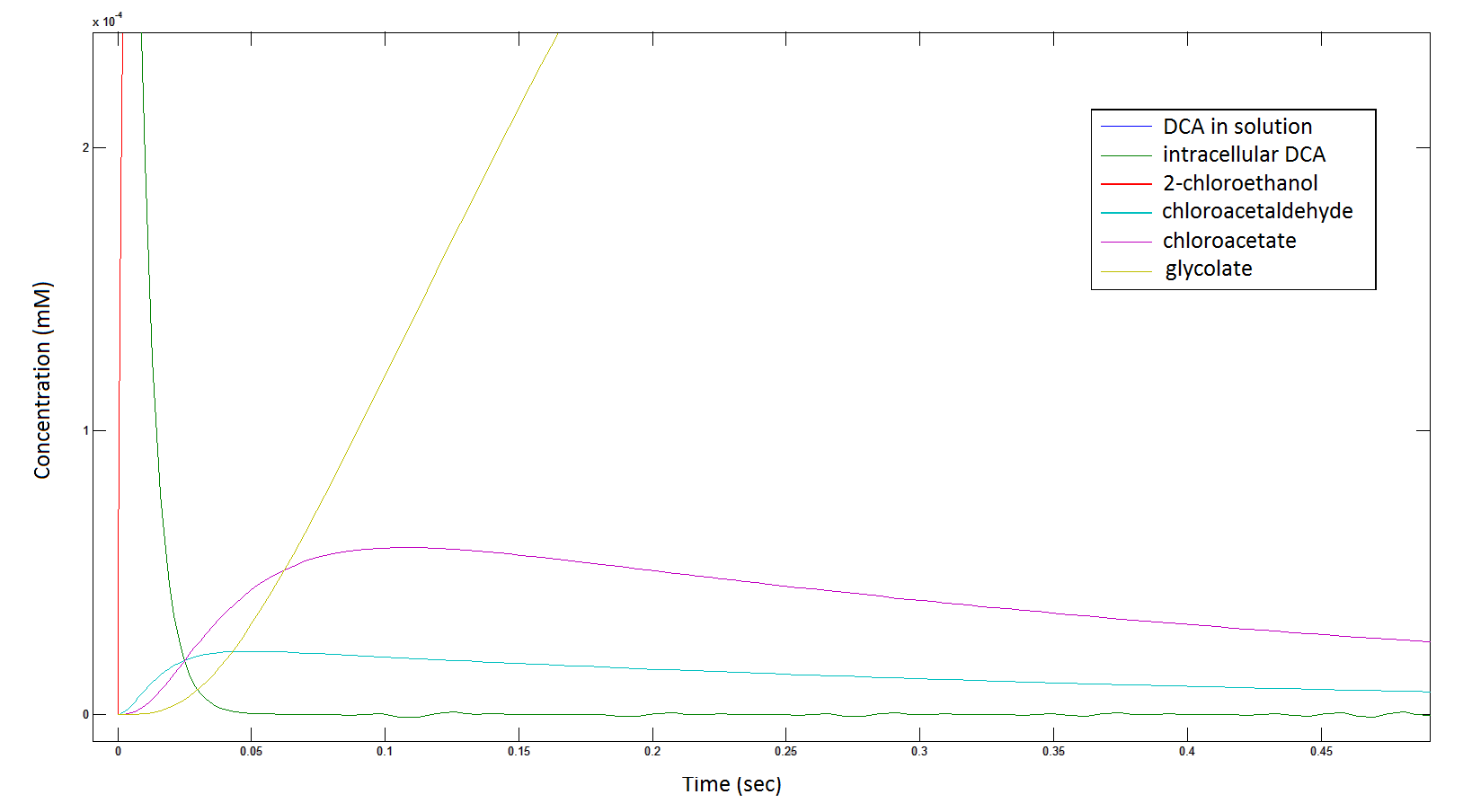
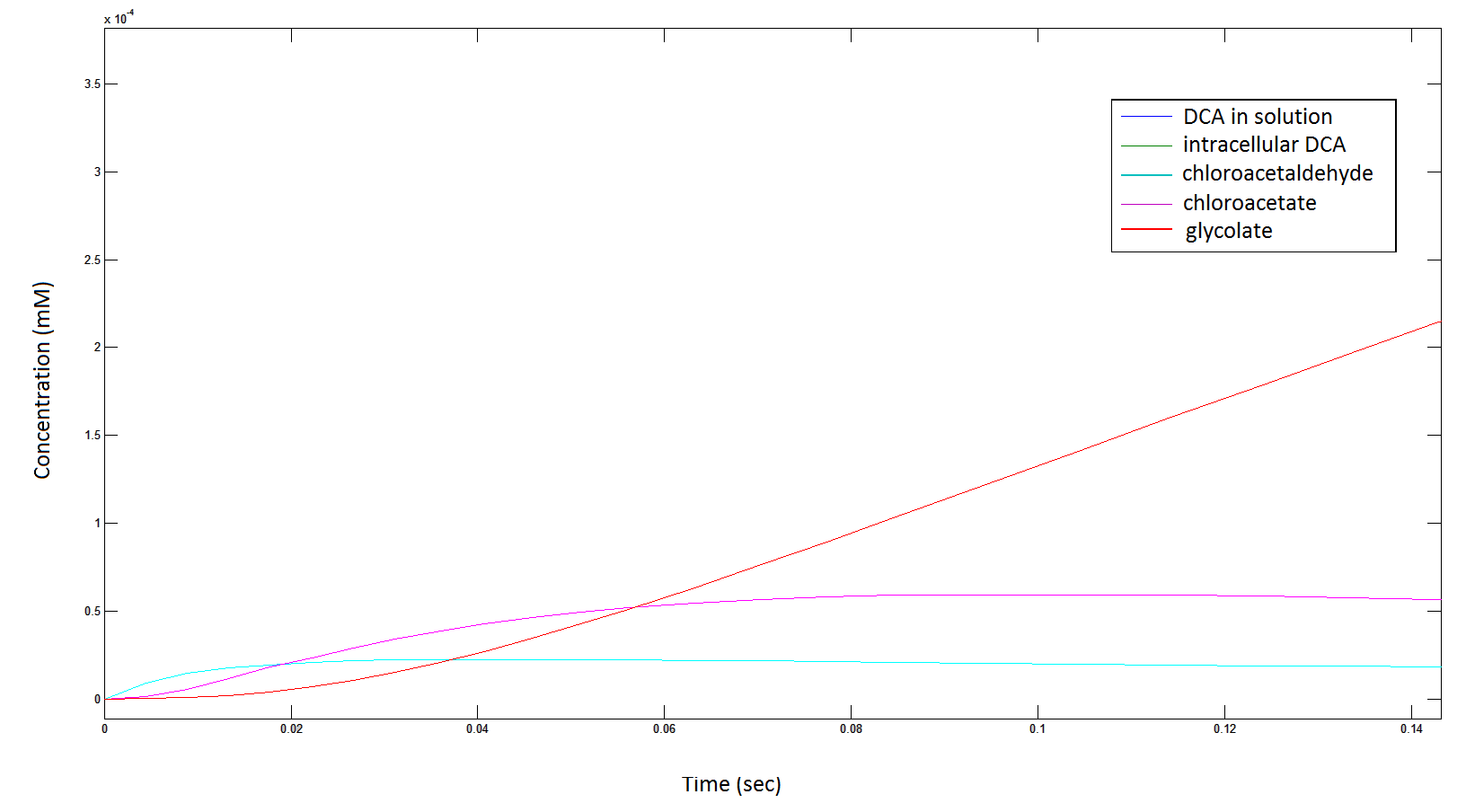
Graph 3: Each line represents the concentration of each of the metabolites . This graph is simply a rescaling of graph 1 and 2.
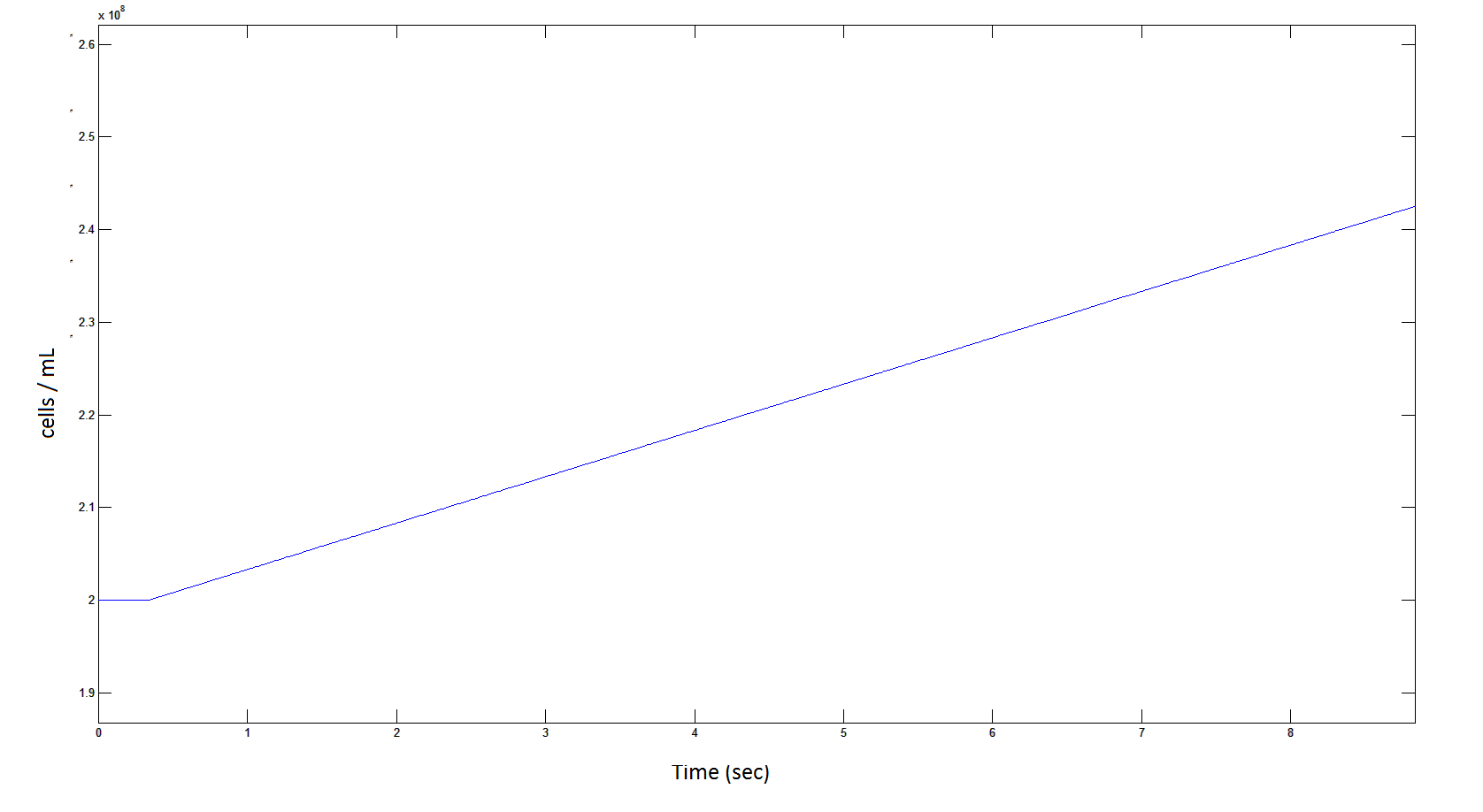
The rate at which the cells grow over time:
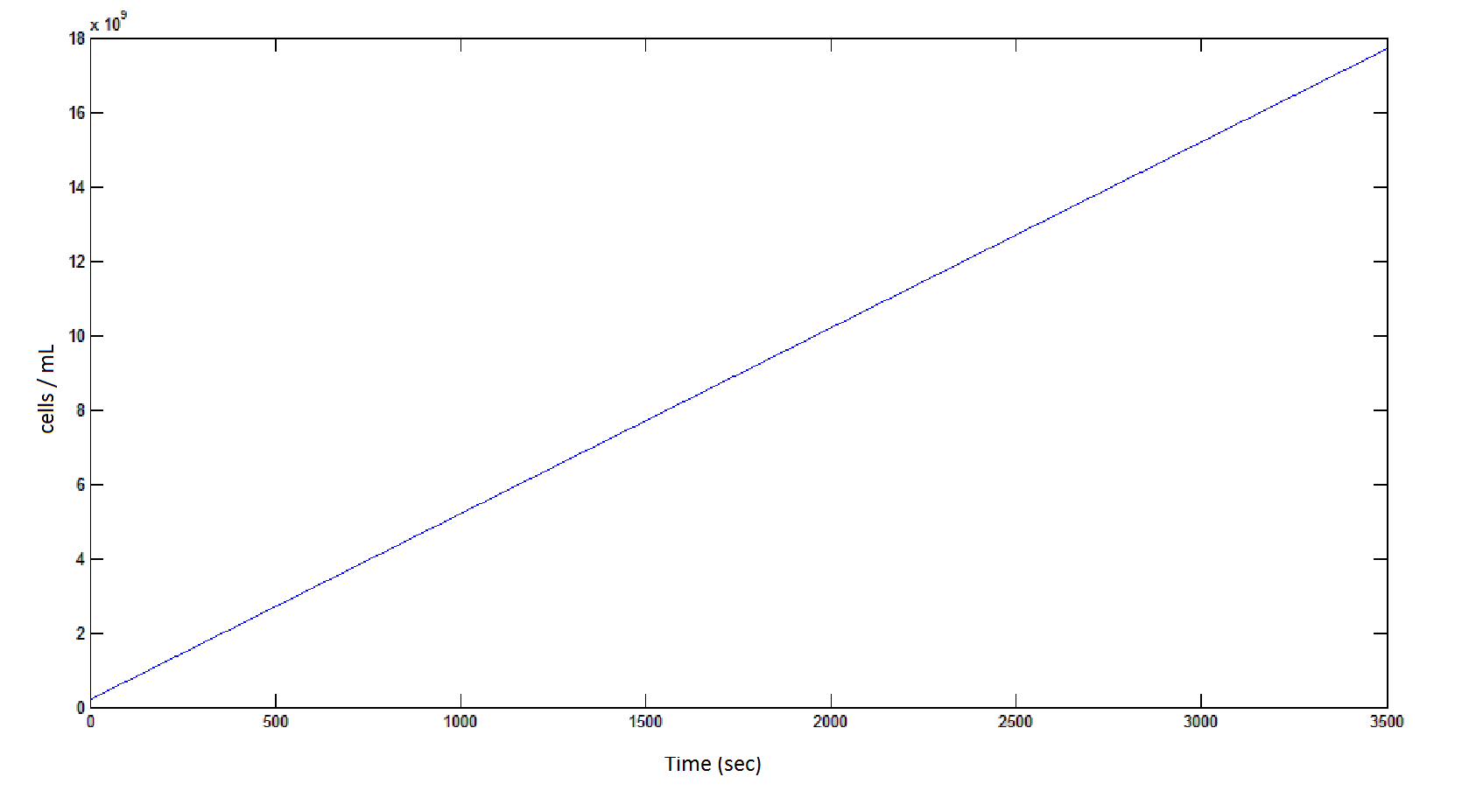
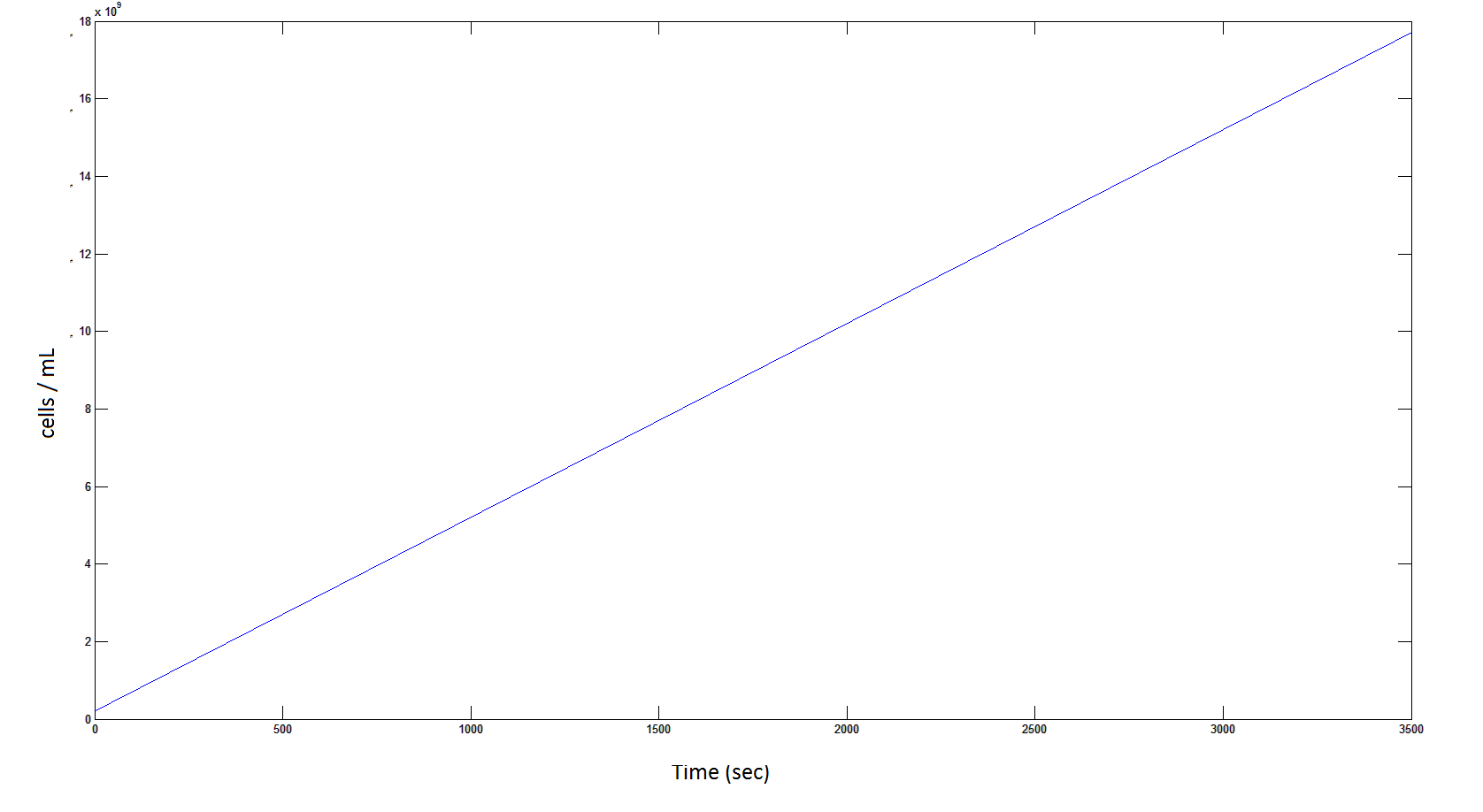
Graph 4: the blue line represents the linear increase of cells due to the presence of intracellular glycolate.
The Non-monooxygenase Pathway
ODE overview:
Raw MATLAB code:
function dy = p450(t,y)
dy=zeros(6,1);
dy(1)= -y(6)*(6*10^(-12))*0.0463067*(y(1)-y(2));
dy(2)= ((6*10^(-12))*0.0463067*(y(1)-y(2))) - 0.0113*25.55*(y(2)/(.12+y(2)));
dy(3) = 0.0113*25.55*(y(2)/(.12+y(2))) - 0.6*25.55*(y(3)/(0.16+y(3)));
dy(4)= 0.6*25.55*(y(3)/(0.16+y(3))) - 25.4*25.55*(y(4)/(20+y(4)));
if y(5) > 2*10^(-10)
dy(5)= 25.4*25.55*(y(4)/(20+y(4))) -1.5789*10^(-10)
else
dy(5) = 25.4*25.55*(y(4)/(20+y(4)))
end
if y(5) > 0.0005
if y(6) > 1.6*10^11
dy(6)=0
else
dy(6) = 5*10^6
end
else
dy(6) = 0
end
end
MATLAB output:
The rate at which DCA is removed in solution:
Graph 5: The blue line represents how the concentration of DCA in solution (extracellular) decreases due to the action of our DCA degraders.
The Rate at which the intracellular concentration of the metabolites change over time:
Graph 6: Each line represents the intracellular concentration of each of the metabolites over time within a single cell. The colour associated with each metabolite is depicted in the figure legend. This graph is simply a rescaling of graph 5.
Rescaling the graph once again: the rate at which the metabolic intermediate change over time.
Graph 7: Again, each line represents the intracellular concentration of each of the metabolites over time within a single cell. This graph is simply a rescaling of graph 5 and 6.
The rate at which the cells grow over time:
Graph 8: The blue line represents the linear increase of the total number of cells due to the presence of intracellular glycolate.
Rescaling Shows a lag effect on cell growth:
Graph 9: The blue line represents the increase of cells due to the presence of intracellular glycolate. This graph is a rescaling of graph 8 to show the inital lag in cellualr growth.
Conclusions:
From Graphs 1 and 5, one can see that 1mM of DCA is removed from solution within roughly 50 minutes when the DCA degrading cells are at a concentration of 2E8 cells/mL.
From Graphs 4, 8 and 9, it is evident that bacterial growth occurs. This growth is due to the production of glycolate, and by comparing graphs 6 and 9, one can see that bacterial growth correlates with glycolate accumulation.
The cytotoxic metabolic intermediate chloroactealdehyde doesn't accumulate to a significant concentration in any of the pathways and is consistently at a negligibly small concentration. From Graphs 3 and 7 one can see that chloroacetaldehyde reaches a maximum concentration of roughly 0.2 mM in both pathways. Chloroacetaldehyde is seen to be metabolised very quickly; this concentration maximum is very short lived where it peaks at roughly 0.03 seconds and returns back to 0 mM by 0.5 seconds. It is expected that chloroacetaldehyde toxicity will not be a problem in our engineered cells.
It is also possible to conclude that the pathways remove DCA at the same rate (through comparing graphs 1 and 5).
References:
[1] Krooshof, G. H., Ridder, I. S., Tepper, A. W., Vos, G. J., Rozeboom, H. J., Kalk, K. H., Dijkstra, B. W. & Janssen, D. B. (1998). Kinetic Analysis and X-ray Structure of Haloalkane Dehalogenase with a Modified Halide-Binding Site. Biochemistry, 37(43), 15013-15023.
[2] Janecki, D. J., Bemis, K. G., Tegeler, T. J., Sanghani, P. C., Zhai, L., Hurley, T. D., Bosron, W. F. & Wang, M. (2007). A multiple reaction monitoring method for absolute quantification of the human liver alcohol dehydrogenase ADH1C1 isoenzyme. Analytical Biochemistry, 369(1), 18-26.
[3] Pandey, A. V. & Flück, C. E. (2013). NADPH P450 oxidoreductase: Structure, function, and pathology of diseases. Pharmacology & Therapeutics, 138(2), 229-254.
[4] van der Ploeg, J., Shmidt, M. P., Landa, A. S., & Janssen, D. B. (1994). Identification of Chloroacetaldehyde Dehydrogenase Involved in 1,2-Dichloroethane Degradation. Applied Environmental Microbiology 60(5), 1599-1605.
[5] van der Ploeg, J., van Hall, G. & Janssen, D. B. (1991) Characterization of the haloacid dehalogenase from Xanthobacter autotrophicus GJ10 and sequencing of the dhlB gene. Journal of Bacteriology, 173(24), 7925-33.
[6] Sinensky, M. I. (1974). Homeoviscous Adaption – A Homeostatic Process that Regulates the Viscosity of Membrane Lipids in Escherichia coli. Proceedings from the National Academy of Science of the United States of America, 71(2), 522-525.
[7] CyberCell Database
[8]
[9]
[10] http://www.dtsc.ca.gov/AssessingRisk/Upload/12dca.pdf
[11] Ishihama, Y., Schmidt, T., Rappsilber, J., Mann, M., Hartl F. U., Kerner, M. J. & Frishman, D. (2008) Protein abundance profiling of the Escherichia coli cytosol. BMC Genomics, 9:102
[12] Lord, J. M. (1972) Glycolate oxidoreductase in Escherichia coli. Biochemica et Biophysica Acta 267:2, 227-327.
 "
"