Team:DTU-Denmark/Notebook/8 August 2013
From 2013.igem.org
8 August 2013
Navigate to the Previous or the Next Entry
Contents |
Lab 208
Main purpose
- PCR for AMO with USER endings.
- Extraction PCR of Nir1
Who was in the lab
Kristian, Julia, Henrike
Procedure
PCR for AMO with USER endings
primers: 17a, 17b
template: gel purified AMO extraction fragment
Program: Standard with 54C annealing temperature and 3:00 extension time
Reaction Mix
| component(per reaction) | without additives | using 5% DMSO | using 1M Betaine |
|---|---|---|---|
| dNTPs | 1uL | 1uL | 1uL |
| HF buffer | 10uL | 10uL | 10uL |
| X7 polymerase | 0.5uL | 0.5uL | 0.5uL |
| MilliQ water | 31.5uL | 29uL | 21.5uL |
| template | 1uL | 1uL | 1uL |
| FW primer | 3uL | 3uL | 3uL |
| RV primer | 3uL | 3uL | 3uL |
| DMSO | - | 2.5uL | - |
| Betaine | - | - | 10uL |
PCR for extraction of Nir1
primers: 41a, 41b
template: colony from plate. One colony was dissolved in 100 uL of MilliQ and 1 uL of this solution was taken as template
program: Touchdown PCR analog to program used on 01-08-2013 to amplify Nir.
Reaction Mix
| component(per reaction) | without additives | using 5% DMSO | using 1M Betaine |
|---|---|---|---|
| dNTPs | 1uL | 1uL | 1uL |
| HF buffer | 10uL | 10uL | 10uL |
| X7 polymerase | 0.5uL | 0.5uL | 0.5uL |
| MilliQ water | 31.5uL | 29uL | 21.5uL |
| template | 1uL | 1uL | 1uL |
| FW primer | 3uL | 3uL | 3uL |
| RV primer | 3uL | 3uL | 3uL |
| DMSO | - | 2.5uL | - |
| Betaine | - | - | 10uL |
Comment
Both PCRs mentioned above were successful when DMSO was added.
Results
Gel on yesterdays PCR
- 1kb ladder
- Yesterdays sample 1
- Yesterdays sample 2
- Yesterdays sample 3
- Yesterdays sample 4
- Neg.
- Neg.
- Nir2
- cycAX Histag
- 1kb ladder
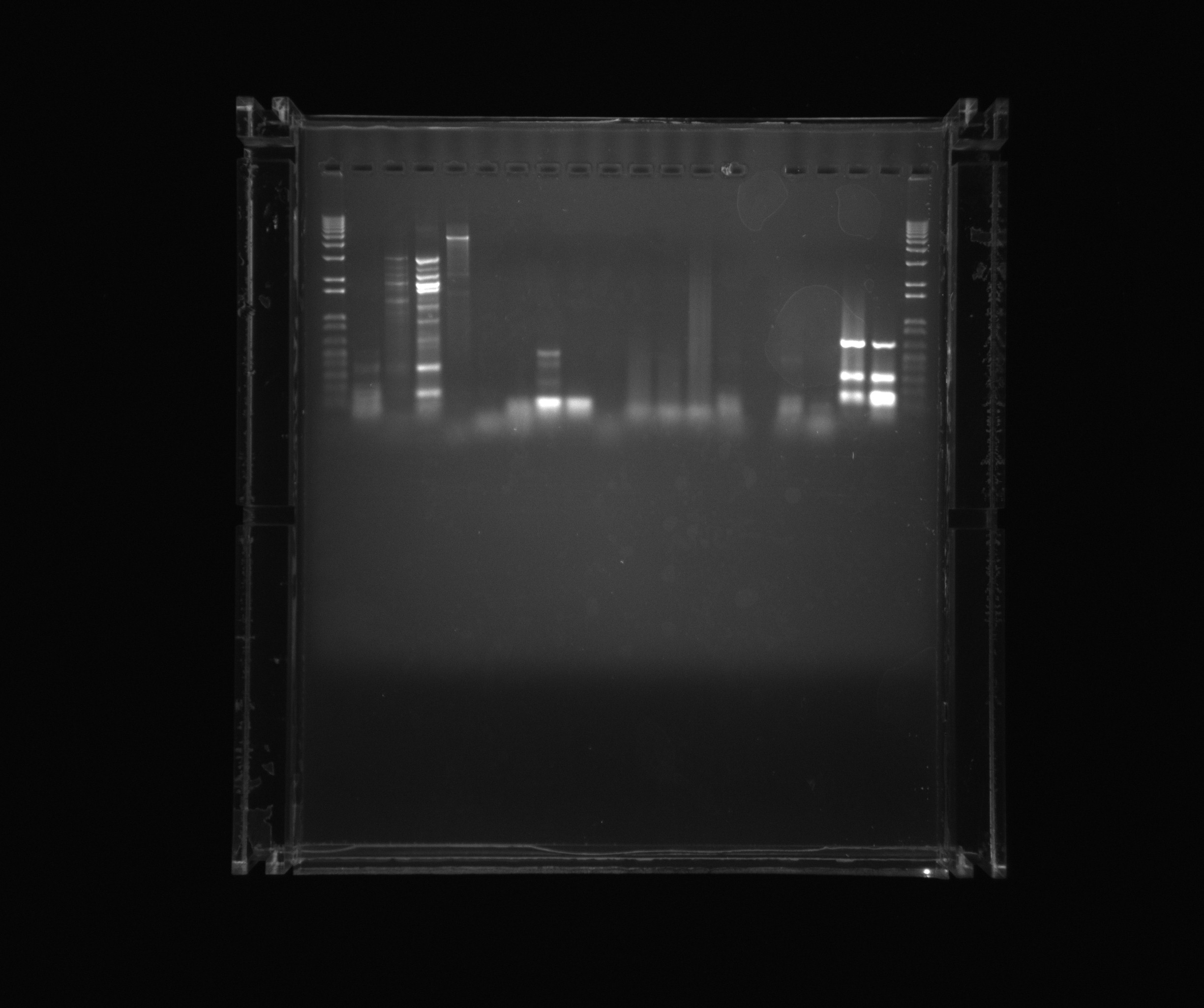
Gel on ON PCR samples
- 1kb ladder
- Nir1
- Nir1 5%DMSO
- Nir1 1M Betaine
- Nir1 3M Betaine
- Nir2 3M Betaine U
- Nir2 1M Betaine U
- Nir2 5%DMSO U
- Nir2 U
- Neg (Nir)
- Ref 0%DMSO
- Ref 2%DMSO
- Ref 5%DMSO
- AMO U
- AMO 2%DMSO U
- Neg (AMO)
- NirG 5%DMSO
- NirG
- 1kb ladder
Gel on todays PCR samples
- 1kb ladder
- Nir Q5 poly
- Nir 2%DMSO Q5 poly
- Nir 3M Betaine Q5 poly
- Nir 5%DMSO Q5 poly
- Nir1 Q5 poly
- Nir1 2%DMSO Q5 poly
- Nir1 5%DMSO Q5 poly
- Nir1 3M Betaine Q5 poly
- AMO USER primers
- AMO +DMSO USER primers
- AMO +Betaine USER primers
- Nir1 DMSO
- Nir1 Betaine
- 1kb ladder
Conclusion
Today's PCRs on AMO and Nir were successful when DMSO was added.
Lab 115
Main purpose
Run Experiment 2
Control measurement
Who was in the lab
Ariadni, Natalia
Procedure
Adjusting the temperature at 37 degrees and calibrating the probes as described in Appendix 5.
Following the protocol Experiment 2
Changing the steps :
19. Add 0.5 ml of nitrite solution and continue by adding 0.5 ml after 10 minutes.
We added 0.5 ml nitrite again and then we took 2 ml of sample, we added then 0.5 ml and afterwards when there was any change in the curve we spiked with 0.4 ml of nitrite. We took 2 ml for sample in the end and we added 0.5 ml of N2O and 1 ml of NO to see if the probes are working properly.
Results
Colorimetric results
Measurements of standard solutions
Ammonium - 43.7 mg/L ( expected 39 mg/L)
Nitrite - < 2 mg/L (X10 diluted) -0.4 mg/L (with no dilution) (expected 0.5 mg/L)
Nitrate - 18.7 mg/L (X10 diluted) (expected 12 mg/L)
Measurement of samples
Ammonium
Measuring range 2-75 mg/L NH4-N
start point- no signal
middle point- signal <2 mg/L
end point- signal 0.7 mg/L
Nitrate
Measuring range 1-25 mg/L NO3-N
start point- no signal
middle point- signal <1 mg/L
end point- signal <1 mg/L
Nitrite
Measuring range 0.02-1 mg/L NO2-N
start point- no signal
middle point- signal 0.43 mg/L
end point- signal 0.79 mg/L
Conclusion
Problems with the NO probe and there were not proper results.
Navigate to the Previous or the Next Entry
 "
"