Team:Newcastle/Parts/l form switch
From 2013.igem.org
| Line 83: | Line 83: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td><img src="https://static.igem.org/mediawiki/2013/5/50/0.4Xylose_GFP_1_1_w1_Phase_Contrast_-GFP-.jpg" alt="Pulpit rock" width=" | + | <td><img src="https://static.igem.org/mediawiki/2013/5/50/0.4Xylose_GFP_1_1_w1_Phase_Contrast_-GFP-.jpg" alt="Pulpit rock" width="268px" height="208px"><br>Figure 2. Plasmid map of our HBsu-sfGFP</td> |
| - | <td><img src="https://static.igem.org/mediawiki/2013/1/1a/%280.5%25%29Xylose_1_1_w1_Phase_Contrast_-RFP-.jpg" alt="Pulpit rock" width=" | + | <td><img src="https://static.igem.org/mediawiki/2013/1/1a/%280.5%25%29Xylose_1_1_w1_Phase_Contrast_-RFP-.jpg" alt="Pulpit rock" width="268px" height="208px"><br>Figure 3. Plasmid map of our HBsu-RFP</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td><img src="https://static.igem.org/mediawiki/2013/e/e8/%280.6%25%29Xylose_1_1_w1_Phase_Contrast_-RFP-.jpg" alt="Pulpit rock" width=" | + | <td><img src="https://static.igem.org/mediawiki/2013/e/e8/%280.6%25%29Xylose_1_1_w1_Phase_Contrast_-RFP-.jpg" alt="Pulpit rock" width="268px" height="208px"><br>Figure 2. Plasmid map of our HBsu-sfGFP</td> |
| - | <td><img src="https://static.igem.org/mediawiki/2013/9/9d/%280.8%25%29Xylose_1_1_w1_Phase_Contrast_-RFP-.jpg" alt="Pulpit rock" width=" | + | <td><img src="https://static.igem.org/mediawiki/2013/9/9d/%280.8%25%29Xylose_1_1_w1_Phase_Contrast_-RFP-.jpg" alt="Pulpit rock" width="268px" height="208px"><br>Figure 3. Plasmid map of our HBsu-RFP</td> |
</tr> | </tr> | ||
</table> | </table> | ||
| Line 95: | Line 95: | ||
<table border="0"> | <table border="0"> | ||
<tr> | <tr> | ||
| - | <td><img src="https://static.igem.org/mediawiki/2013/8/83/168_rod.jpg" alt="Pulpit rock" width=" | + | <td><img src="https://static.igem.org/mediawiki/2013/8/83/168_rod.jpg" alt="Pulpit rock" width="268px" height="208px"><br><i>Figure 1. Plasmid map of Pmutin4 without insert</i></td> |
| - | <td><img src="https://static.igem.org/mediawiki/2013/0/0e/168_L-form.jpg" alt="Pulpit rock" width=" | + | <td><img src="https://static.igem.org/mediawiki/2013/0/0e/168_L-form.jpg" alt="Pulpit rock" width="268px" height="208px"><br><i>Figure 1. Plasmid map of Pmutin4 without insert</i></td> |
</tr> | </tr> | ||
</table> | </table> | ||
| Line 102: | Line 102: | ||
<table border="0"> | <table border="0"> | ||
<tr> | <tr> | ||
| - | <td><img src="https://static.igem.org/mediawiki/2013/2/2c/BSB1_rod.jpg" alt="Pulpit rock" width=" | + | <td><img src="https://static.igem.org/mediawiki/2013/2/2c/BSB1_rod.jpg" alt="Pulpit rock" width="268px" height="208px"><br><i>Figure 1. Plasmid map of Pmutin4 without insert</i></td> |
| - | <td><img src="https://static.igem.org/mediawiki/2013/4/45/BSB1_L-form.jpg" alt="Pulpit rock" width=" | + | <td><img src="https://static.igem.org/mediawiki/2013/4/45/BSB1_L-form.jpg" alt="Pulpit rock" width="268px" height="208px"><br><i>Figure 1. Plasmid map of Pmutin4 without insert</i></td> |
</tr> | </tr> | ||
</table> | </table> | ||
Revision as of 12:52, 22 September 2013

Contents |
Rod to L-form Switch BioBrick
Purpose and justification
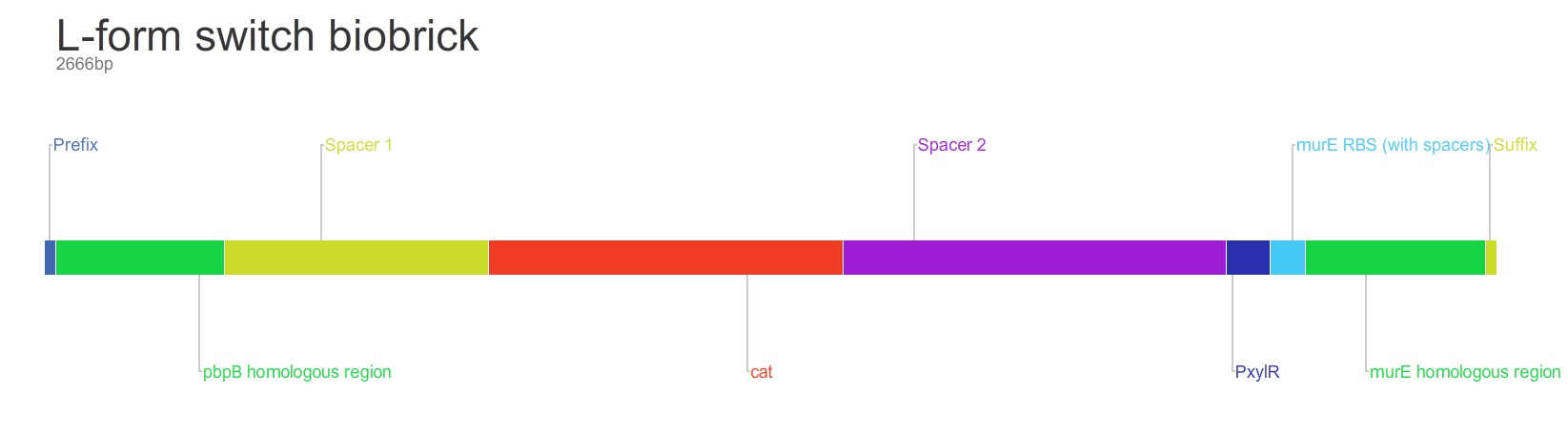
The purpose of this main ‘keystone’ biobrick is to facilitate the switching of Bacillus subtilis cells from rod-shape to L-form, wall-less cells. Furthermore, this ‘switch’ enables L-form cells to return to rod-shape when required. This is facilitated through the introduction of a xylose-controlled promoter (PxylR) upstream of the murE gene, which is involved in the biosynthesis of peptidoglycan. The presence, or absence of a cell wall can be controlled through the presence, or absence of xylose, respectively. The antibiotic resistance marker chloramphenicol acetyl-transferase (cat) is upstream of this promoter region to allow for the selection of cells in which this system is integrated.
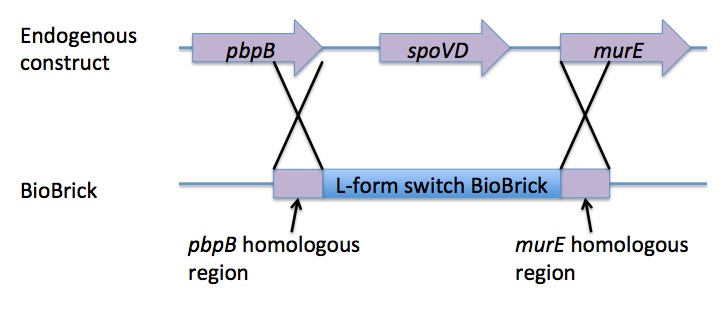
Homologous recombination allows this BioBrick to be integrated into the chromosome of B. subtilis. The cat and PxylR sequences are flanked by sequences that are homologous with regions of the B. subtilis chromosome, which allows for insertion of the BioBrick at the intended loci. ~300bp at the 5’ end of the BioBrick is homologous with the end of the pbpB gene. Similarly ~300bp at the 3’ end of the biobrick is homologous with the start of the murE gene.
Integration of the BioBrick facilitates xylose-mediated control over the expression of murE. This cascades through biosynthetic pathways to enable control over peptidoglycan synthesis and thus control over cell wall production. Simply, in B. subtilis cells that have integrated the BioBrick into their chromosome, only in the presence of xylose is the cell wall produced. When xylose is not present cells lose their cell wall and survivors adopt the L-form phenotype.
The L-form BioBrick BBa_K1185000 can be found on the Registry of Standard Biological Parts.

Design
The L-form switch BioBrick is based upon the regulatory sequence controlling expression of murE in B. subtilis LR2 - a strain capable of L-form state. In this B. subtilis strain the peptidoglycan synthesis pathway required for the cell wall can be disrupted through control of murE expression - responsible for the synthesis of enzymes involved in peptidoglycan precursor biosynthesis. A xylose-contolled PxylR promoter upstream of murE facilitates this control. Upstream of this promoter the LR2 strain also contains a cat gene - conferring chloramphenicol resistance. These genetic components differentiate the B. subtilis LR2 chromosome from that of B. subtilis 168. On the LR2 chromosome these genetic components take the place of the spoVD gene (involved in sporulation) in B. subtilis 168. It is these genetic elements that have been utilised to create a BioBrick that facilitates the switching of B. subtilis cells from rod state to L-form, and back again. The flanking regions of the cat gene and the PxylR promoter - part of the pbpB coding sequence at the 5' end of this region of DNA and part of the murE coding sequence at the 3' end - are also of vital importance, allowing for integration of the BioBrick into the chromosome of B. subtilis via homologous recombination.
In order to package the genetic elements within the B. subtilis chromosome that enable this strain to transition from rod to L-form, the full sequence of the region between the pbpB and the murE genes had to be identified. The sequence for the L-form switch region was obtained through a de novo assembly (using Sequencher 5.1 DNA sequencing software) of sequence data gained from sequencing between the pbpB and murE genes on the B. subtilis LR2 chromosome (using designed primers) with reads produced by high throughput sequencing of the full B. subtilis LR2 genome. In particular, reads that did not assemble when using B. subtilis 168 as a reference genome were used in this assembly. The primers that were designed to sequence the region between the pbpB and murE genes on the B. subtilis LR2 chromosome allowed sequencing from both the 5’ and the 3' ends of the desired sequence. The design of the primers allowed for sequencing to include the final 309bp at the 3’ end of the pbpB coding sequence (upstream of the L-form switch region), as well as the sequence of the 331bp at the 5’ end of the murE coding sequence (downstream, and under the control, of the L-form switch). Once fully sequenced, this region of DNA was able to be synthesised once compliant with the BioBrick RFC[10] standard.
To allow the L-form switch BioBrick to be submitted to the iGEM registry of standard biological parts it needed to comply with BioBrick RFC[10] standard. In order to comply with this standard the BioBrick RFC[10] prefix and suffix sequences were added to the synthetic construct sequence using Sequencher 5.1 DNA sequencing software. Two EcoRI restriction sites and a PstI restriction site that were present in the sequence were removed through single nucleotide changes using Gene Designer 2.0 - these restriction sites violated the terms of the RFC[10] standard. Base changes were chosen to optimise the altered codons (in the open reading frame of coding sequences) for codon bias in B. subtilis. The altered genetic sequence was then able to be synthesised.
Modelling
Modelling biosynthesis of peptidoglycan, specifically involving manipulation of the murE gene. Currently undergoing completion…
Construction
The designed BioBrick was synthesised by DNA synthesis company DNA 2.0. The components within the BioBrick were as follows: -
Parts:
- Biobrick prefix – RFC[10] standard.
- 309bp of the end of the pbpB coding sequence.
- Spacer.
- Reverse and complement of chloramphenicol acetyl-transferase (cat) coding sequence (including native ribosome binding site (RBS) and promoter).
- PxylR promoter.
- Spacer.
- RBS binding site for murE.
- Spacer.
- 331bp of the start of murE coding sequence.
- Biobrick suffix – RFC[10] standard.
The synthesised BioBrick was then inserted into the pSB1C3 plasmid - the only plasmid acceptable by iGEM for part submission.
Cloning and integration
The pSB1C3 plasmid containing the L-form switch BioBrick returned from DNA 2.0 was amplified in E.coli. The E. coli competent cell preparation and E. coli transformation protocols were used to transform this plasmid into E. coli cells for cloning. Transformant cells were incubated in order to clone the transformed plasmids. Then the cloned plasmids were extracted and transformed into B. subtilis. Integration of the BioBrick from the vector plasmid to the host chromosome was facilitated through homologous recombination with crossover events within the pbpB and murE genes.
Testing and Characterisation
Transformants will be selected through growth on LB+Chloramphenicol+0.5-0.8% xylose media. Only those which took up the DNA and integrate the DNA will survive.
To characterise this BioBrick, the B. subtilis is to be grown on a media without xylose and then the morphology of the cells will be checked under the microscope. Successfully transformed B. subtilis cells should have converted to L-form state (lost their cell walls). These are recognisable due to their rounded and non-uniform morphology. Cells may also vary greatly in size.
 Figure 1. Plasmid map of Pmutin4 without insert |
 Figure 1. Plasmid map of Pmutin4 without insert |
 Figure 2. Plasmid map of our HBsu-sfGFP |
 Figure 3. Plasmid map of our HBsu-RFP |
 Figure 2. Plasmid map of our HBsu-sfGFP |
 Figure 3. Plasmid map of our HBsu-RFP |
 Figure 1. Plasmid map of Pmutin4 without insert |
 Figure 1. Plasmid map of Pmutin4 without insert |
 Figure 1. Plasmid map of Pmutin4 without insert |
 Figure 1. Plasmid map of Pmutin4 without insert |
There are 2 main approaches to do this:
Liquid media
Lysozyme protoplasting method
 "
"