Team:Newcastle/Project/plants
From 2013.igem.org
| Line 149: | Line 149: | ||
Besides methods of imaging, alternative staining methods were also considered to enhance the images of L-forms in plants. As autofluorescent chloroplasts are normally present in the upper parts of the plant, certain red fluorescent dyes can provide useful counter fluors for GFP. An example of this would be propium iodide and FM 1-43 | Besides methods of imaging, alternative staining methods were also considered to enhance the images of L-forms in plants. As autofluorescent chloroplasts are normally present in the upper parts of the plant, certain red fluorescent dyes can provide useful counter fluors for GFP. An example of this would be propium iodide and FM 1-43 | ||
| - | ===Propium Iodide=== | + | ====Propium Iodide==== |
Propium iodide is red fluorescent and can be detected using a filter set suitable for Texas Red fuorescence. It can be applied to live seedlings in water to specifically label root cell walls forming an outline of the cell. As our L-forms do not contain cell walls, the staining of the plant cell walls will provide an extremely good contrast, revealing the localization of the bacteria. | Propium iodide is red fluorescent and can be detected using a filter set suitable for Texas Red fuorescence. It can be applied to live seedlings in water to specifically label root cell walls forming an outline of the cell. As our L-forms do not contain cell walls, the staining of the plant cell walls will provide an extremely good contrast, revealing the localization of the bacteria. | ||
| - | ===FM 1-43=== | + | ====FM 1-43==== |
FM1-43 is an orange stain that stains the plasma membrane in roots and shoot tissues. As the imaging of the seedling is mainly at the base of the shoot, this dye provides a good contrast as well, contrasting the fluorescence emitting from the GFP labelled L-form. | FM1-43 is an orange stain that stains the plasma membrane in roots and shoot tissues. As the imaging of the seedling is mainly at the base of the shoot, this dye provides a good contrast as well, contrasting the fluorescence emitting from the GFP labelled L-form. | ||
Revision as of 12:26, 2 October 2013

Contents |
L-forms in Plants
In nature L-forms and plants can exist in a symbiotic relationship as plants provide an osmotically suitable environment. L-forms can confer benefits to their host including inhibition of fungal pathogenesis. This lead us to consider whether it would be possible to create an artificial symbiotic relationship between plants and L-forms. We washed Brassica pekinensis (Chinese Cabbage) seedlings in a solution of green fluorescent protein (GFP) labelled L-forms, allowing the plants to take up the bacteria. We then viewed our L-forms inside the plant using light and confocal microscopy.
In the future, L-forms could be engineered to supply nutrients or other useful compounds, such as pesticides, to plants. This could potentially increase crop yield and create more nutritious plants.
L-forms are osmotically sensitive, giving the ethical advantage that they lyse if they escape from their host plant into the environment. This makes them much less likely to escape into the environment than cell walled bacteria.
Purpose
As the world’s population continues growing there is an ever greater need to improve crop yield. There are already worldwide food shortages and things will only get worse. Furthermore climate change will reduce the area of suitable agricultural land and the effectiveness of many commonly used crops, therefore it is necessary to maintain and increase the productivity of agricultural land wherever possible.
We propose that L-forms could be modified to produce benefits to plants - producing nutrients or protection against pathogens. Research has already shown prevention of fungal pathogenesis in Chinese Cabbage and Strawberry plants which have been exposed to B. subtilis L-forms. The mechanism by which this works is not yet understood, but could we harness the anti-fungal nature of these micro-organisms? It could be possible to take nitrogen fixing bacteria such as Rhizobia, turn them into L-forms and inoculate them into plants to produce non-leguminous nitrogen fixing crops. These sorts of ideas could have a big impact on agriculture, preventing crop spoilage and increasing crop yield. As the world population rises so will the demand for food. Producing enough food is not a new problem for humans, yet this novel form of bacteria may be part of the solution.
Using L-forms may appear an unnecessary step- why not just modify the plant cells? First, bacteria are significantly easier to manipulate and genetically modify than plant cells, which are very complex. There are also bacteria genes, beneficial to plants, which would not be functional if transformed into a GM plant. Furthermore, there are ethical boundaries associated with modifying plants. GM plants could well outcompete native species, spreading away from farmland and into the wild, becoming weeds and reducing biodiversity. They could also crossbreed with other strains.
Using non-L-form bacteria also has multiple potential pitfalls. Kill switches are never 100% safe as there is always the risk of mutations that turn off these genes, resulting in bacteria able to proliferate freely. L-forms however are incredibly osmotically sensitive, unable to survive outside of plants. Therefore they could be introduced into the environment - if they do leave the plants, they will very quickly pop due to water entering through osmosis, and a lack of a cell wall to counteract the resulting increased internal pressure. We have shown this experimentally using water extracted from soil.
Inoculating plants with L-forms is therefore safer than inoculating with regular bacteria and also safer than directly modifying plants.
Our Objectives
Our primary objective was to show that L-forms can be grown inside Chinese Cabbage. However in the process of doing this we also produced a protocol for the inoculation of L-forms into Chinese Cabbage that can be used by future iGEM teams working with L-forms. We also wanted to characterise the osmotic instability of L-forms, as this instability makes L-forms a more attractive proposition to inoculate into crops than cell-walled bacteria. Finally we wished to investigate the laws governing the release of genetically modified organisms.
Figure 1. Chinese Cabbage seedlings inoculated with L-forms growing on a Murishage and Skoog plate.
Methods
In order to prove that L-forms can grow in plants we soaked germinating Chinese Cabbage seedlings in a solution of GFP-labelled L-forms. We then replanted the seedlings and viewed our L-forms via light microscopy before using confocal microscopy to generate better quality images. Our protocol is modified from Tsomlexoglou et al. (2003): -
Inoculating L-forms into Chinese Cabbage
- Rinse seeds for 2 minutes in 70% (v/v) ethanol.
- Soak seeds for 10 minutes in 20% (v/v) Milton’s sterilizing fluid.
- Wash seeds thoroughly five times in sterile distilled water and leave in final wash for fifteen minutes.
- Place seeds in (9cm) Petri dishes (20-25 seeds per dish) containing Murashige and Skoog (M and S) basal medium, solidified with 0.8% (w/v) agar no.1 (Oxoid, UK) and incubate at 25°C in the dark until radicals just appear (this should take 21-24hr for Chinese cabbage).
- Use spectrophotometry to produce a L-form suspension containing approximately 107 CFU ml-1 (OD600: approx. 0.7).
- Select seeds with radicals 1-2mm in length and soak in the bacterial suspension (20 seeds per 10ml) for 3 hours at 25°C, gently shaking the seeds by hand every 30 minutes. Treat some seedlings with 5% (w/v) mannitol instead of L-forms to act as a control.
- Wash seeds ten times in distilled water.
- Replant plants on M and S agar plates.
- Incubate seeds in sunlight for between 24 hours and 7 days at 25°C.
- The plants are ready to be viewed using microscopy.
The washing of seeds in ethanol and Milton’s sterilizing fluid is carried out to kill any bacteria residing on the surface of the seed. M and S medium is a growth media designed for the cultivation of plants. Washing the seeds in distilled water after soaking them in bacterial solution lyses any L-forms not inside the plant. We carried out our microscopy 4 days after the seeds were soaked in L-forms, to co-inside with when Tsomlexoglou et al. (2003) carried out PCR to prove the presence of L-forms in their Chinese Cabbage.
Alternative Methods Considered
Before deciding to use GFP-labelled L-forms, we considered a couple of alternative ways to detect L-forms in plants: -
β-glucuronidase assay
In order to prove that L-forms can grow in plants, we considered transforming B. subtillis strain LR2 with the gusA reporter gene coding for β-glucuronidase. We would have removed gusA BioBrick from its plasmid backbone and attach it to another plasmid containing aprE for homologous recombination and kanamycin resistance for selection. We would then have transformed B. subtillis with the new transformation vector. The bacteria that have taken up the plasmid can would have been selected for via kanamycin resistance. A transformation vector would have been used rather than a normal plasmid so that gusA would integrate through homologous recombination into the bacteria’s chromosome, securing the expressing of the gusA.
The β-glucuronidase produced by the L-forms in plants would cleave the colourless substrate 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (X-Gluc) to produce a blue product allowing the L-form distribution in plant tissue to be observed. We considered this method after reading a paper by Tsomlexoglou et al. (2003).
Enzyme-linked immunosorbent assay (ELISA)
We considered producing an ELISA to detect L-forms. We could have generated antibodies against an L-form specific antigen. Alternatively we considered introducing a novel gene into L-forms to produce a novel antigen to generate antibodies against. However this would have involved using animals to generate poly-clonal antibodies. We decided this raised ethical issues and was too time consuming to complete in our ten week placement. We considered this method after reading a paper by Ferguson et al. (2003).
DAPI Staining
4',6-diamidino-2-phenylindole (DAPI) can be used to stain both bacterial and plant DNA fluorescent blue. For this reason we considered using DAPI to counter-stain the plant genome and inoculating the seedlings with GFP-labelled L-forms so we can see both the plant nucleus and our L-forms. Alternatively we could have stained GFP-labelled L-forms with DAPI so that when viewing L-forms with confocal microscopy we could check if they fluoresce blue as well as green. This would add further evidence that we had successfully inoculated L-forms into Chinese Cabbage.
PCR
Tsomlexoglou et al. (2003) used the polymerase chain reaction (PCR) to detect L-forms containing the gusA gene in four day old Chinese Cabbage. Primers specific to the gusA gene were used as this gene was specific to the genetically modified L-forms. We considered using primers specific to our GFP-labelled L-forms to carry out PCR. We chose not to carry out this method due to time constraints and we wanted a more aesthetic detection method.
Results
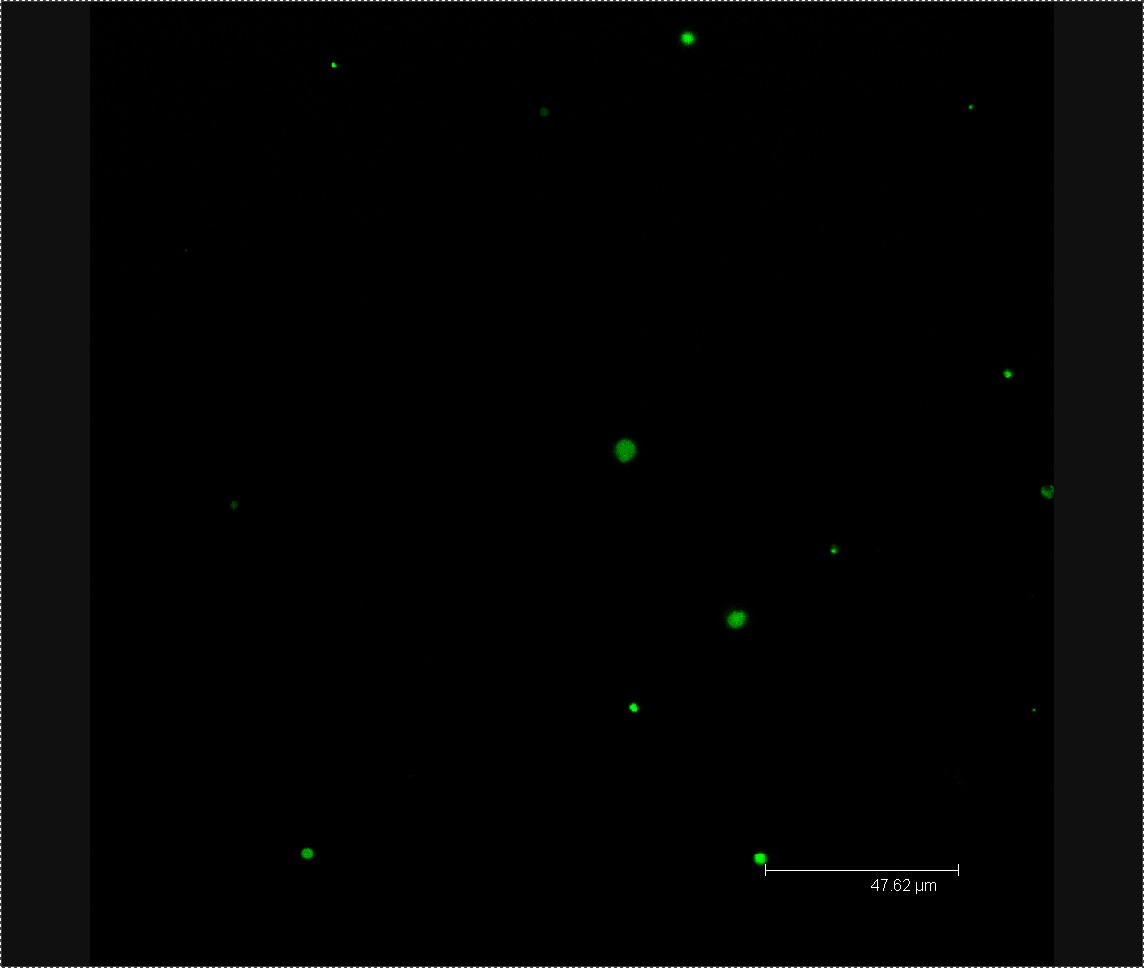
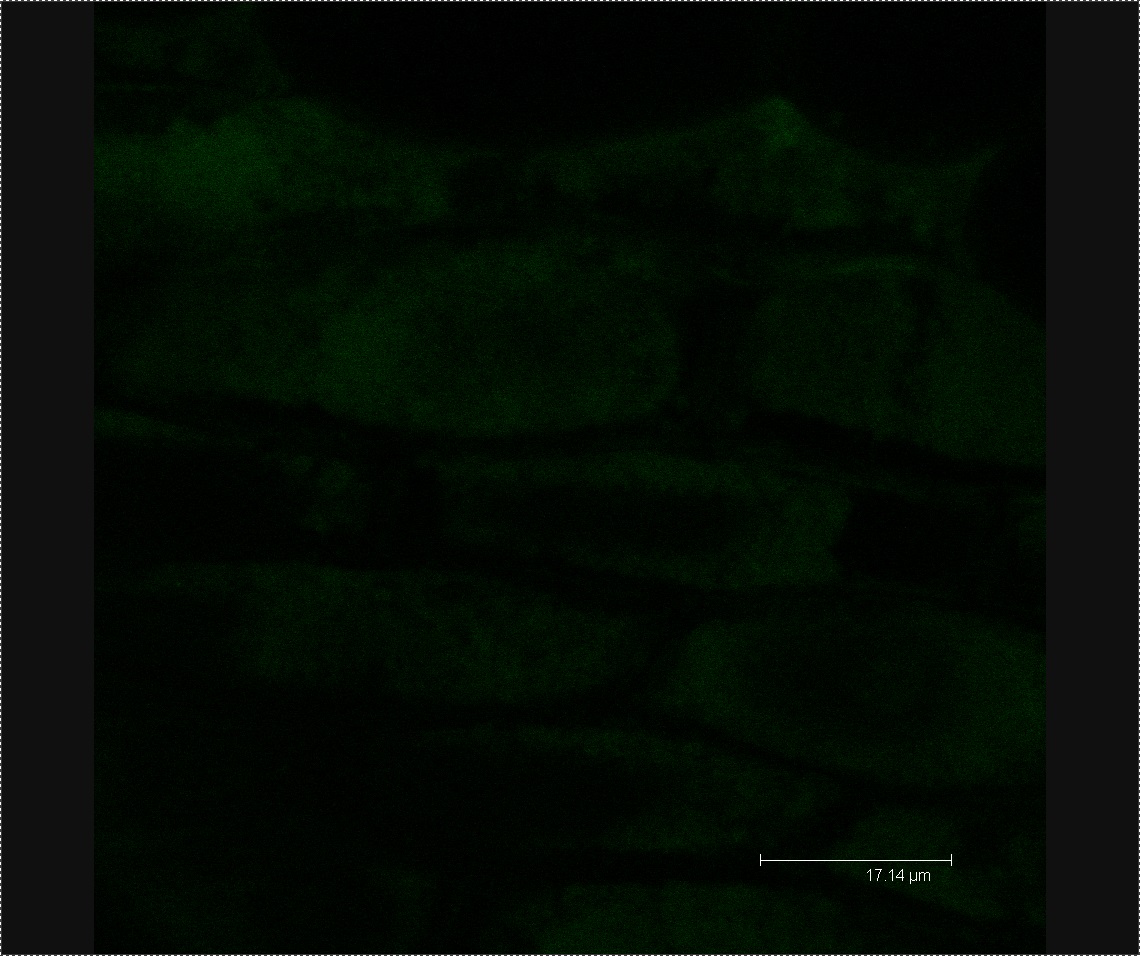
Four days after inoculating our Chinese Cabbage with L-forms, we checked to see if the bacteria had found their way into the seedlings. We checked our L-form-washed plants and our mannitol-washed (negative control) plants using confocal microscopy to see if there was any green fluorescence of the corresponding size and shape to be L-forms. We were able to generate images of our L-form-washed seedlings showing green fluorescent blobs ranging in size from approximatly 2 to 6 microns as seen in Figure 2. The fluorescent shapes are of the correct size and appearance to be L-forms. In our negative control we could find no such green fluorescence, only images showing plant cells as shown in Figure 3. These results show that the Chinese Cabbage that we treated contained our GFP-labelled L-forms.


Figure 2. Confocal microscopy of L-form washed plants. This image shows a brightfield image of plant cells, with an image of the green fluorescence overlaid on top. The green blobs ranging in size from approximatly 2 to 6 microns are L-forms.


Figure 3. Confocal microscopy of negative control plants. This image shows a brightfield image of plant cells, with an image of the green fluorescence overlaid on top. There are no L-forms present in the image, the green fluorescence is sparse and not of the correct appearance to be L-forms.
These results show that we were successful in producing plants containing genetically engineered L-forms. In the future other synthetic biologists could adopt this delivery method to inoculate Chinese Cabbage with L-forms engineered to provide substances to the plant including nitrates, antifungals or plant hormones.
Results Log
09/08/13
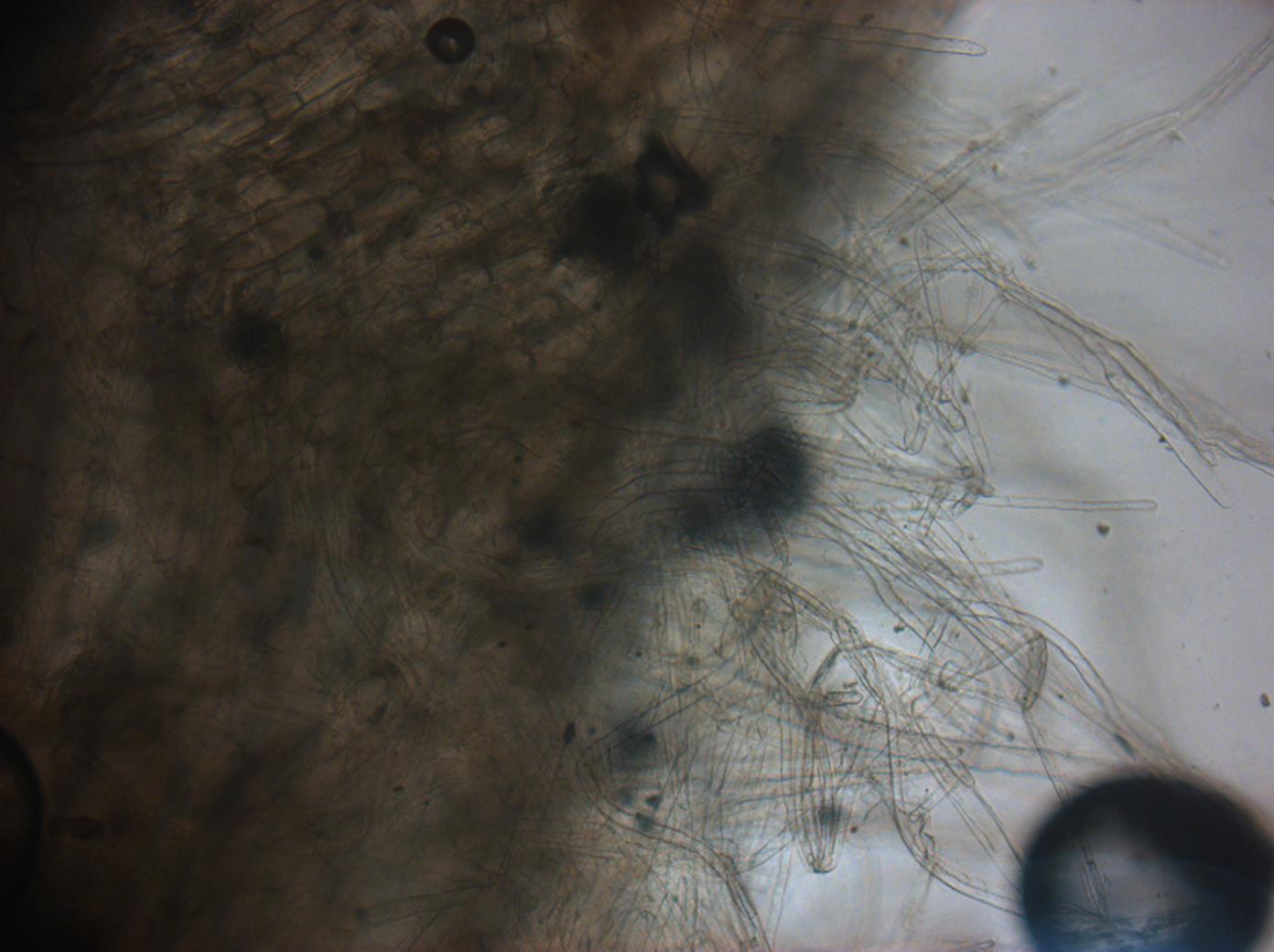
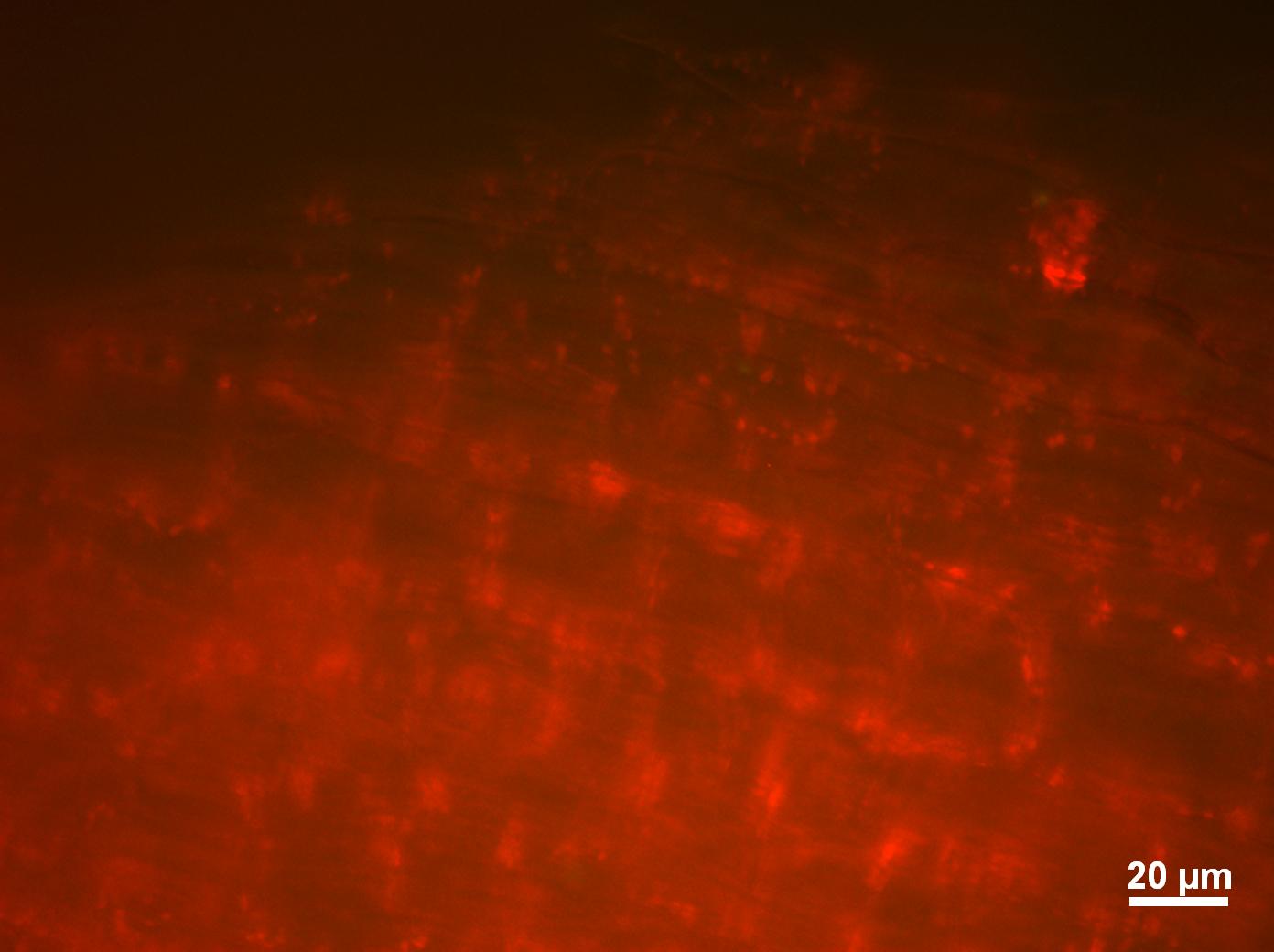
Today we viewed the seedlings, that we had washed with L-forms yesterday,using bright field and fluorescence microscopy. Unfortunately we could not see anything that looked convincing as a possible L-form, for that reason we have decided to move onto using confocal microscopy to view our seedlings. Hopefully using a more this more sophisticated technique we may be able to see some fluorescing L-forms. Figure one shows a brightfield image of our chinese cabbage roots under low magnification and Figure 2. shows cell from the chinese cabbage root expressing background red fluorescence.
Figure 4. Brightfield chinese cabbage root hairs under low magnification.
Figure 5. Chinese cabbage root cells expressing background red fluorescence.
14/08/13
Today we viewed our plants using confocal microscopy to try and detect any GFP-labelled bacteria. After viewing a few seeds washed in L-form solution under the microscope, we found a green fluorescing dot in the correct size range to be an L-form. No green dots were found in the rod cell or mannitol controls. Following these results we have decided to use confocal microscopy four days after washing our plants in L-form solution, rather than after one. This co-insides with when Tsomlexoglou et al. used detected L-forms in their chinese cabbage using PCR.
Figure 6. Possible L-form inside Chinese cabbage. The bright green dot is the correct size to be an L-form. The green fluorescence image used to detect the L-form is superimposed onto a bright-field image of plant matter.
Figure 7. L-forms from the solution used to wash the Chinese cabbage.
23/08/13
We viewed 7 different Chinese cabbage seedlings washed with L-forms under the confocal microscope. In three of these plants we found clusters of green fluorescent dots about 0.5µm in diameter. We found no clusters of green fluorescent dots in three seedlings soaked in the mannitol control.
Figure 5. Cluster of L-forms inside a Chinese cabbage seedling.
Figure 6. Image of mannitol-soaked control exhibiting faint green auto-fluorescence, allowing us to see the outline of plant cells.
30/08/13
On this day we performed more confocal microscopy and were able to see clusters of L-forms inside our seedlings.


Figure 7. Confocal microscopy of L-form washed plants. This image shows a brightfield image of plant cells, with an image of the green fluorescence overlaid on top. The green blobs ranging in size from approximatly 2 to 6 microns are L-forms.
Imaging Alternatives for the Future
Some difficulties faced while imaging the plants by confocal microscopy were:
1)There are deep layers of highly refractile cell wall and aqueous cytosol in the plant cell wall
2)The plant had autofluorescent and light scattering constituents
To address these difficulties, two methods were considered:
1)To fix and clear the tissue with a high-refractive index mounting medium
2)To directly image living tissue using suitably corrected microscope optics
The second method was looked into at greater depth as it would be difficult to effectively clear plant wholemounts without causing artifacts or risking the loss of GFP fluorescence. Following the second method, we mounted our seedlings in water under glass coverslips. This method of direct visualization of GFP fluorescence in living tissue will solve problems to do with fixation or staining artifacts. The images obtained from this method will give good clarity.
Besides methods of imaging, alternative staining methods were also considered to enhance the images of L-forms in plants. As autofluorescent chloroplasts are normally present in the upper parts of the plant, certain red fluorescent dyes can provide useful counter fluors for GFP. An example of this would be propium iodide and FM 1-43
Propium Iodide
Propium iodide is red fluorescent and can be detected using a filter set suitable for Texas Red fuorescence. It can be applied to live seedlings in water to specifically label root cell walls forming an outline of the cell. As our L-forms do not contain cell walls, the staining of the plant cell walls will provide an extremely good contrast, revealing the localization of the bacteria.
FM 1-43
FM1-43 is an orange stain that stains the plasma membrane in roots and shoot tissues. As the imaging of the seedling is mainly at the base of the shoot, this dye provides a good contrast as well, contrasting the fluorescence emitting from the GFP labelled L-form.
Although, these staining methods were not used due to the time constraint the team had, if given more time, better images may have been obtained, showing in detail the localization of the L-forms.
References
Paton, A.M. (1987) 'L-forms: evolution or revolution?', J Appl Bacteriol, 63(5), pp. 365-71.
 "
"