Team:Paris Bettencourt/Project/Sabotage
From 2013.igem.org
| Line 71: | Line 71: | ||
In nature, non-lytic filamentous bacteriophages are quite good at spreading genetic elements in bacterial populations and we thus imediately thought about them as vectors of choice. However being infected by a phage represent a huge burden for an individual bacteria which we thought would be detrimental for our construct to be able to maintain itself long enough in a population for this system to be clinically relevant. <br><br>Therefore we choose to use a phagemid/helper system which is composed of two mobile interacting elements. The « heavy » elements of this system is an M13 phage which genome contains an altered packaging signal thus reducing the probability of packaging in a protein capside and escaping from the cell. Its role is to produce capside proteins which will in fact be used by a « light » element called phagemid which is a normal plasmid harboring a packaging signal. | In nature, non-lytic filamentous bacteriophages are quite good at spreading genetic elements in bacterial populations and we thus imediately thought about them as vectors of choice. However being infected by a phage represent a huge burden for an individual bacteria which we thought would be detrimental for our construct to be able to maintain itself long enough in a population for this system to be clinically relevant. <br><br>Therefore we choose to use a phagemid/helper system which is composed of two mobile interacting elements. The « heavy » elements of this system is an M13 phage which genome contains an altered packaging signal thus reducing the probability of packaging in a protein capside and escaping from the cell. Its role is to produce capside proteins which will in fact be used by a « light » element called phagemid which is a normal plasmid harboring a packaging signal. | ||
</p> | </p> | ||
| - | < | + | </div> |
| + | <div class="rightparagraph"> | ||
<p> | <p> | ||
<br>As a result a cell containing both elements will produce and secrete a lot of phagemid encapsulated plus some helper phages from time to time. We expect such a system to infect with light elements the majority of cells in a population and to be able to spread and maintain itself thanks to a small number of coinfected cells harboring both light (phagemid) and heavy (helper phage) elements transforming them into phage-producing cells.<br><br> | <br>As a result a cell containing both elements will produce and secrete a lot of phagemid encapsulated plus some helper phages from time to time. We expect such a system to infect with light elements the majority of cells in a population and to be able to spread and maintain itself thanks to a small number of coinfected cells harboring both light (phagemid) and heavy (helper phage) elements transforming them into phage-producing cells.<br><br> | ||
Our silencing device is loaded on the light element in order to spread it efficiently. As the post-transcriptional regulation we are using only rely on RNA and doesn’t require synthesis of any protein, its cost for the cell will be very low. Moreover producing protective proteins against antibiotics is costly for the cell and lower its fitness in an antibiotic free environment, we thus expect our silencing device to be a temporary relief for the infected cell which should avoid early counterselection dynamics | Our silencing device is loaded on the light element in order to spread it efficiently. As the post-transcriptional regulation we are using only rely on RNA and doesn’t require synthesis of any protein, its cost for the cell will be very low. Moreover producing protective proteins against antibiotics is costly for the cell and lower its fitness in an antibiotic free environment, we thus expect our silencing device to be a temporary relief for the infected cell which should avoid early counterselection dynamics | ||
</p> | </p> | ||
| + | </div> | ||
| + | <div style="clear: both;"></div> | ||
<br> | <br> | ||
| + | <div class="leftparagraph"> | ||
<p> | <p> | ||
<b>Design of a sequential killing strategy</b> <br> | <b>Design of a sequential killing strategy</b> <br> | ||
| Line 82: | Line 86: | ||
The experimental set up consisted of a sequential strategy, a phage infection followed by a treatment with antibiotics. | The experimental set up consisted of a sequential strategy, a phage infection followed by a treatment with antibiotics. | ||
</p> | </p> | ||
| + | </div> | ||
<br> | <br> | ||
<div class="rightparagraph"> | <div class="rightparagraph"> | ||
Revision as of 19:34, 4 October 2013

Background
One of the main concern about tuberculosis today is the emergence of antibiotic resistant strain
Results
- Construction and characterization of phagemids coding for small RNA targeting antibiotic resistance proteins
- successful conversion of antibiotic resistant population of E. coli to a sensitive state
BioBricks
Aims
Our objective is to make an antibiotic-resistant bacterial population sensitive again to those same antibiotics.
Introduction
As resistance against antibiotics are growing and pharmaceutical pipelines are drying up, we decided to investigate a new strategy based on specific silencing of the genes responsible for resistance through bioengineered stealth bacteriophages. The silencing of the genes is obtained with a simple and modular system of tailormade small RNAs and the spreading of this construct in a bacterial population is based on an autonomous phagemid/helper phage system. We demonstrated the validity of this trojan horse strategy by converting back to a sensitive state populations of bacteria initially resistant to antibiotics like chloramphenicol or kanamycin.
 Figure 1. The Trojan Horse Strategy :
Figure 1. The Trojan Horse Strategy :
Design
Design a silencing device
We choose a straight forward strategy to force bacteria to stop producing those proteins by targeting the mRNA coding for those proteins with tailormade small RNAs.In order to design our silencing device (small RNA) we used the protocol described in Na et al 2013 and inserted a 24bp sequence complementary to the RBS area of the target genes inside the scaffold sequence that allow the stabilization of the hybridization. We designed three sRNA respectively targeting Kanamycine resistance gene of Duet Vector pACYc), Chloramphenicol ones ()and lac Z
.
 Figure 2. The sRNA anti-Cm
Figure 2. The sRNA anti-Cm
Design a genetic element that spread in a population
In nature, non-lytic filamentous bacteriophages are quite good at spreading genetic elements in bacterial populations and we thus imediately thought about them as vectors of choice. However being infected by a phage represent a huge burden for an individual bacteria which we thought would be detrimental for our construct to be able to maintain itself long enough in a population for this system to be clinically relevant.
Therefore we choose to use a phagemid/helper system which is composed of two mobile interacting elements. The « heavy » elements of this system is an M13 phage which genome contains an altered packaging signal thus reducing the probability of packaging in a protein capside and escaping from the cell. Its role is to produce capside proteins which will in fact be used by a « light » element called phagemid which is a normal plasmid harboring a packaging signal.
As a result a cell containing both elements will produce and secrete a lot of phagemid encapsulated plus some helper phages from time to time. We expect such a system to infect with light elements the majority of cells in a population and to be able to spread and maintain itself thanks to a small number of coinfected cells harboring both light (phagemid) and heavy (helper phage) elements transforming them into phage-producing cells.
Our silencing device is loaded on the light element in order to spread it efficiently. As the post-transcriptional regulation we are using only rely on RNA and doesn’t require synthesis of any protein, its cost for the cell will be very low. Moreover producing protective proteins against antibiotics is costly for the cell and lower its fitness in an antibiotic free environment, we thus expect our silencing device to be a temporary relief for the infected cell which should avoid early counterselection dynamics
Design of a sequential killing strategy
To perform our proof of concept experiments, our targeted genes were different antibiotic resistance genes on commercial plasmids that were transformed in MG1655 cells.
The experimental set up consisted of a sequential strategy, a phage infection followed by a treatment with antibiotics.
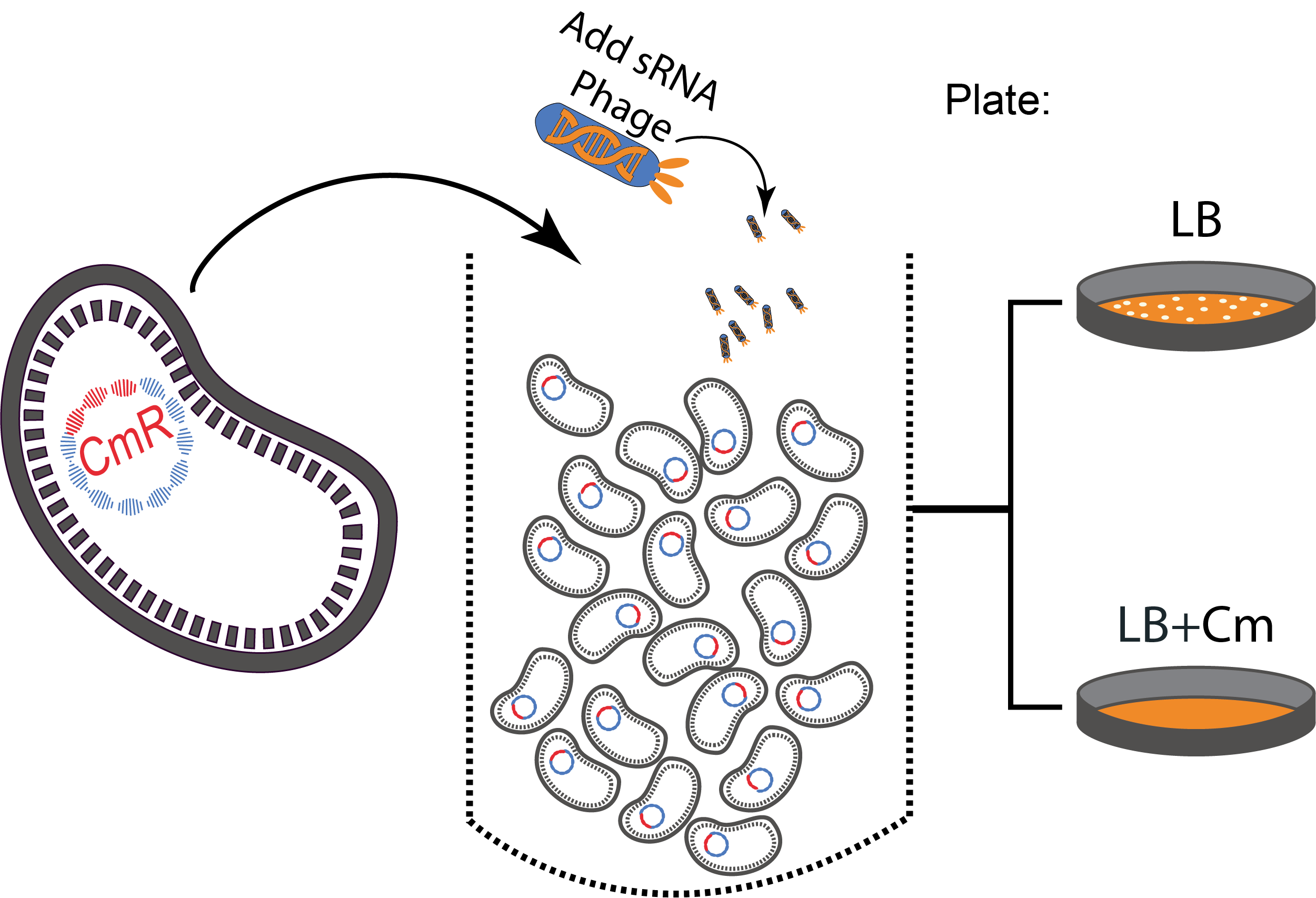 Figure 3. A sequential strategy
Figure 3. A sequential strategy
Characterization of the phagemid system
 Figure 4. Characterization of the phagemid helper system
Figure 4. Characterization of the phagemid helper system
Making a Chloramphenicol resistant E. Coli population sensitive to Chloramphenicol
Results, generalization fro Kan
 Figure 5. Killing Chloramphenicol resistant E. Coli population with Chloramphenicol
Figure 5. Killing Chloramphenicol resistant E. Coli population with Chloramphenicol
Charaterization of the origin of resistance
 Figure 6. Origin of resistance to the sequential killing
Figure 6. Origin of resistance to the sequential killing
Conclusion and perspectives
Several sRNA on the same plasmid : BLABLABLA
Building a library of sRNA : BLABLABLA
Possible Applications : BLABLABLA
 "
"







 +33 1 44 41 25 22/25
+33 1 44 41 25 22/25


