Team:Tokyo Tech/Experiment/Crosstalk Circumvention Assay
From 2013.igem.org
Crosstalk Circumvention Assay
1. Introduction
Of course, if you want to avoid crosstalk, you have to search idea for improvement for crosstalk circumvention. However, you must make conditions without affecting system wide, or without changing the basis of system in the idea for improvement. This is because "crosstalk circumvention by changing system-wide" is the same meaning as saying "crosstalk circumvention only in that circuit". For example, when you want to avoid crosstalk in the circuit on the basis of toggle switch system, you should think that it is nonsense to avoid crosstalk by abandoning toggle switch system. You should think it's nice to avoid crosstalk without abandoning toggle switch system. In short, I hope crosstalk circumvention system is applicable to various circuits. Based on this view, we planned the following idea and experimentation.
2. Summary of the experiment
Our purpose is to check whether or not lux/tet hybrid promoter would be repressed when 3OC12HSL-LasR complex and protein TetR exist. We tried to compare the frequency of crosstalk in the presence or absence of the TetR inhibitor aTc.
We prepared six conditions as follow.
E-1) Culture containing crosstalk circumvention system cell with 3OC6HSL induction E-2) Culture containing crosstalk circumvention system cell with 3OC12HSL induction E-3) Culture containing crosstalk circumvention system cell with DMSO ( no induction) F-1) Culture containing crosstalk circumvention system cell with 3OC6HSL and aTc induction F-2) Culture containing crosstalk circumvention system cell with 3OC12HSL and aTc induction F-3) Culture containing crosstalk circumvention system cell with DMSO and aTc (no induction) Positive control and negative control are similarly operated.
3. Results of Prediction
aTc weakens the affinity of TetR for tetO. Therefore, if the GFP expression level of the cells that we added 3OC6HSL and aTc were higher than that of the cells we added only 3OC6HSL, it is proved that lux/tet hybrid promoter is repressed by TetR. Similarly, if the level of GFP expression of the cells we added 3OC12HSL and aTc was higher than that of the cells we added only 3OC12HSL, it is proved that the crosstalk can be suppressed by TetR and the hybrid promoter.
4. Materials and Methods
4-1. Construction
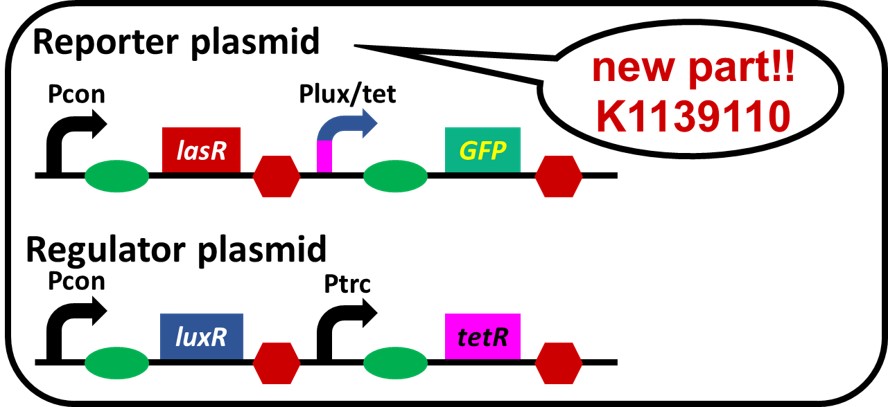
On the previous assay we confirmed crosstalk, which 3OC12HSL-LasR complex activates not only las promoter but also lux promoter, too (Gray KM et al., 1994). We came up with idea that crosstalk can be suppressed if lux/tet hybrid promoter is inhibited by protein TetR only when 3OC12HSL-LasR complex is around. We replaced lux promoter with lux/tet hybrid promoter. What we have to do in this step is to confirm lux/tet hybrid promoter works correctly as a part of the system. Therefore, we tried to make a simple crosstalk circumvention system (Fig. 3-2-1). luxR, tetR, and lasR are under a constitutive promoter.
Because of the unexpected restriction enzyme binding site on the regulator plasmid, we had to place luxR and tetR on the regulator plasmid, and lux/tet hybrid promoter, GFP and lasR on the reporter plasmid (Fig. 3-2-2).
To construct the circuit in above figure (Fig. 3-2-2), we ligated Pcon-RBS-lasR-TT (K553003) and Plux/tet-RBS-GFP-TT (K934025) as the reporter plasmid. We used Pcon-RBS-luxR-TT-Ptrc-RBS-tetR-TT as the regulator plasmid.
Reporter: pSB6A1-Pcon-lasR-Plux/tet-GFP / Regulator: pSB3K3-Pc-luxR-Ptrc-tetR (JM2.300)...sample
pSB6A1-Ptet-GFP (JM2.300)…positive control
pSB6A1-Promoterless-GFP (JM2.300)…negative control
4-2. Strain
・JM2.300
4-3. Protocol
1. O/N -> FC -> Induction
1.1 Prepare overnight culture of each cell (GFP posi, GFP nega, sample) at 37°C for 12 h.
(=> O/N)
1.2 Take 30 microL (from GFP posi, GFP nega, sample) of the overnight culture of inducer cell into LB (3 mL) + antibiotics (Amp 50 microg/mL+ Kan 30 microg/mL).
(=> Fresh Culture)
1.3 Incubate the flesh culture of cells (GFP posi, GFP nega, sample) until the observed OD600 reaches around 0.50.
1.4 Take 30 microL each cell suspensions (GFP posi , GFP nega , sample) into
LB (3 mL) + antibiotics (Amp 50 microg/mL + Kan 30 microg/mL)
+ 5 microM 3OC6HSL (3 microL),LB (3 mL) + antibiotics (Amp 50 microg/mL + Kan 30 microg/mL)
+ 5 microM 3OC6HSL (3 microL) + 0.05 microg/mL aTc (3 microL),LB (3 mL) + antibiotics (Amp 50 microg/mL + Kan 30 microg/mL)
+ 5 microM 3OC12HSL (3 microL),LB (3 mL) + antibiotics (Amp 50 microg/mlL+ Kan 30 microg/mL)
+ 5 microM 3OC12HSL (3 μL)+ 0.05 mg/mL aTc (3 microL),LB (3 mL) + antibiotics (Amp 50 microg/mL + Kan 30 microg/mL)
+ 5 microM DMSO (3 microL) andLB (3 mL) + antibiotics (Amp 50 microg/mL + Kan 30 microg/mL)
+ 5 microM DMSO (3 microL) + 0.05 mg/mL aTc (3 microL).
1.5 Incubate all samples (6 samples X 6 kinds of culture = 36 samples) for another 4h at 37°C.
(=> Induction)
2. Measurement (Flow cytometer)
2.1 Measure all samples' OD600.
2.2 Dilute all samples with 1X PBS to keep OD600 in the range from 0.2 to 0.5.
2.3 Take 1 mL (from all samples) into disposal tube (for flow cytometer).
2.4 Centrifuge them at 9000 g, 4°C, 1 min. and take their supernatant away.
2.5 Suspend all samples with 1 mL 1X PBS.
2.6 Measure all samples.
2.7 Save and organize data.
5. Result of the assay
From this chart (Fig. 3-2-3), it turns out the following. lux/tet hybrid promoter is repressed by TetR in the presence of 3OC6HSL-LuxR complex . Similarly, lux/tet hybrid promoter is repressed by TetR in the presence of 3OC12HSL-LasR complex. In brief, results from E-1) and F-1) are similar to those from E-2) and F-2). Now, therefore, the crosstalk circumvention experiment succeeded.
6. Reference
1. Gray KM, Passador L (1994) Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. Journal of bacteriology 176(10): 3076–3080.
 "
"