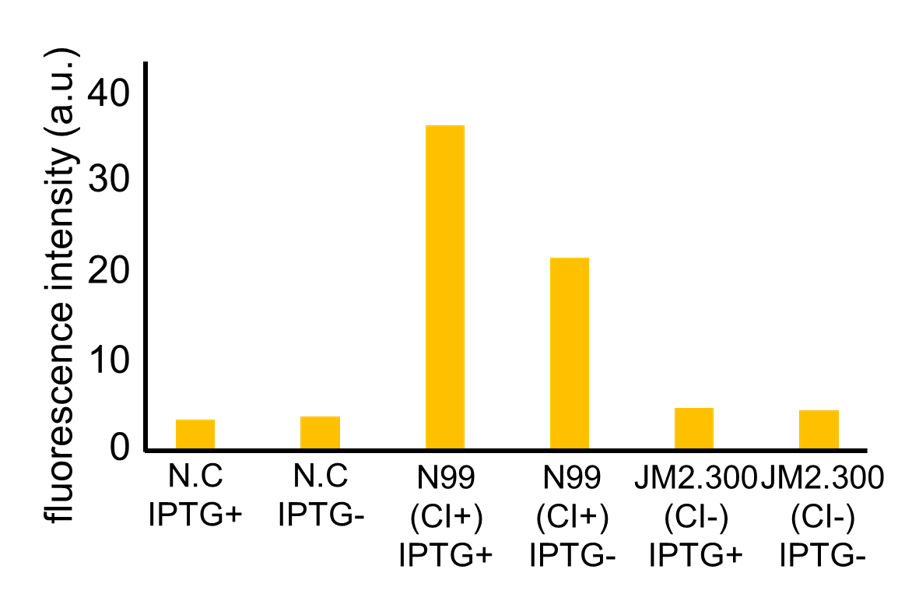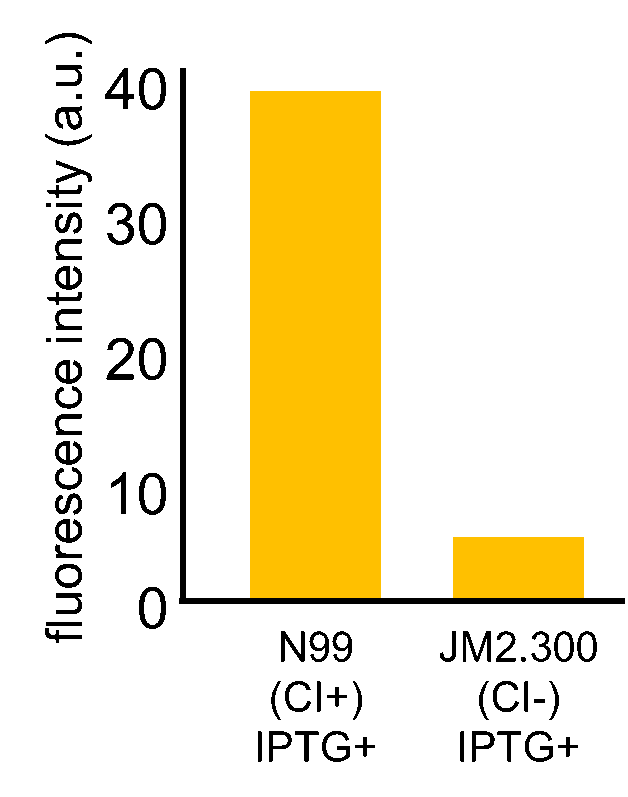Team:Tokyo Tech/Experiment/RM-lac Hybrid Promoter Assay
From 2013.igem.org
Contents |
rm/lac Hybrid Promoter Assay
1. Introduction
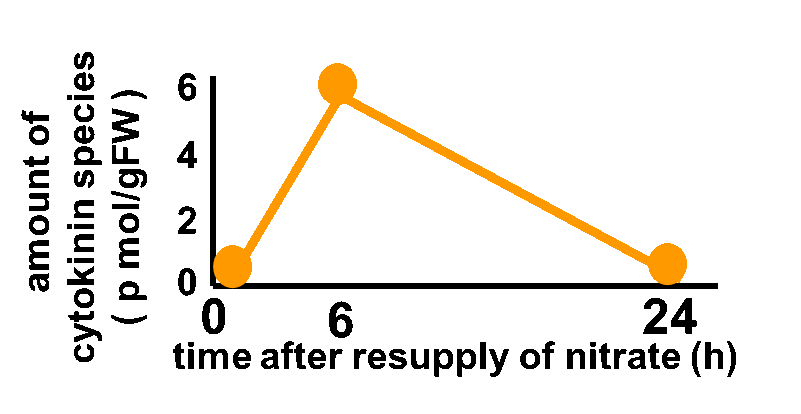
In order to achieve the natural plants’ temporal pattern for producing plant hormones in E. coli, we introduced an incoherent feed forward loop, including a new hybrid promoter part (BBa_K1139150). Plants produce their hormones transiently rather than steadily (Takei et al., 2001) (Fig. 3-7-1). Moreover, continuous overexpression of hormones can be harmful to plants (K. Thiman, 1937). Thus, we thought that it should be important to achieve this transient temporal pattern for producing plant hormones in E. coli. By applying the incoherent feed forward loop, the transient pulse of gene expression can be generated (Basu. S et al., 2003, S. Mangan et al., 2006).
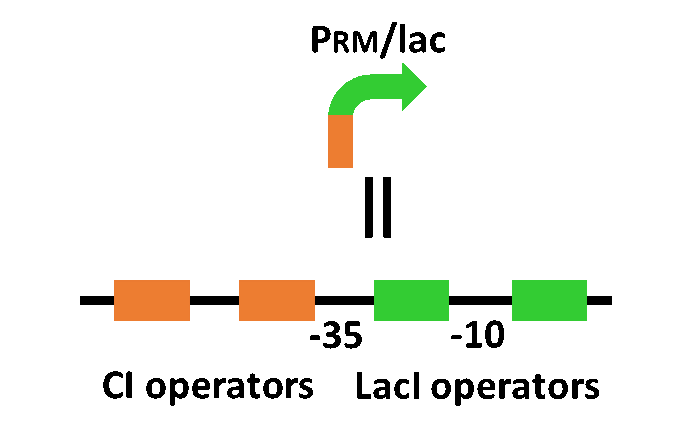
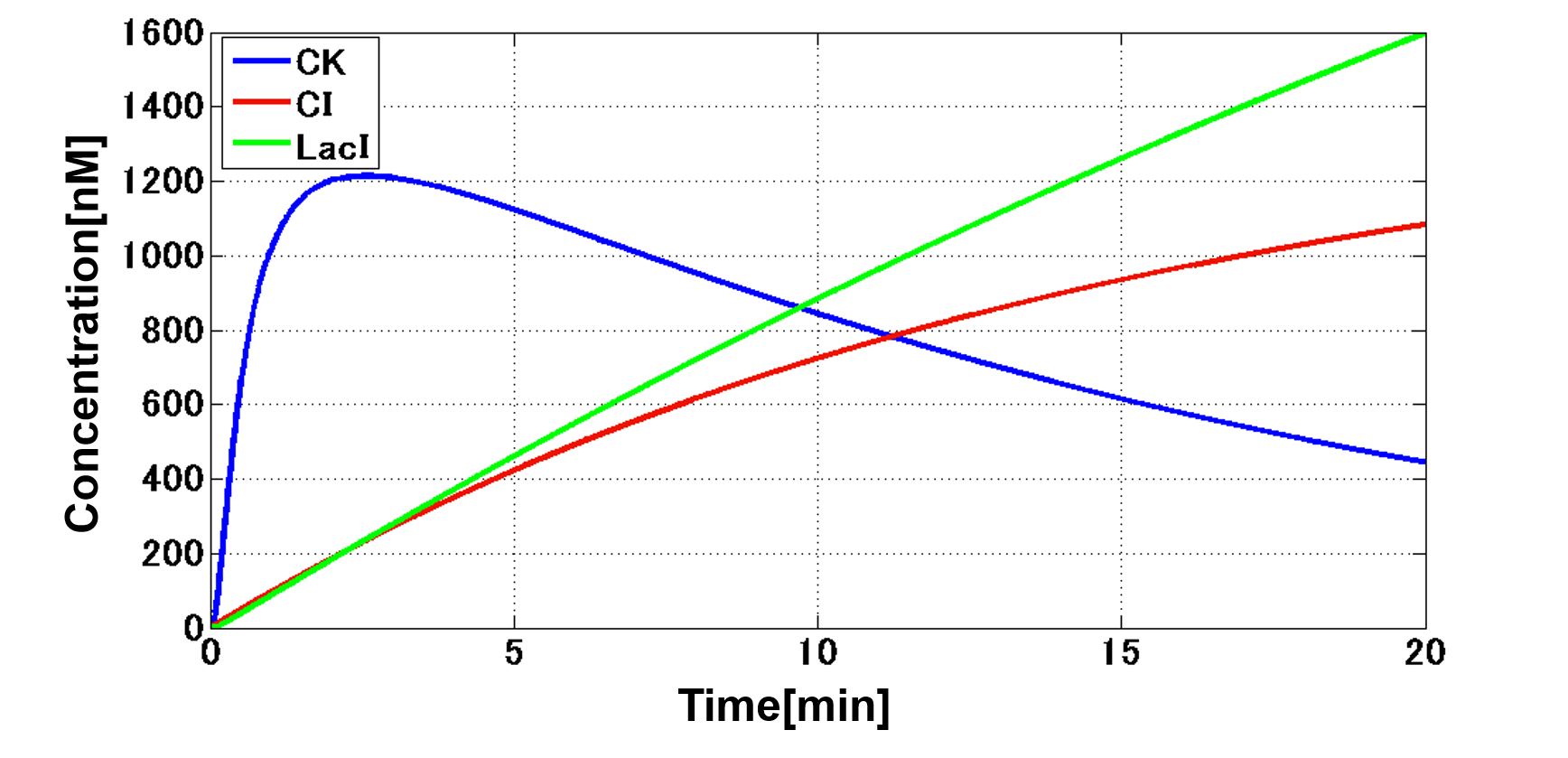
The system of our designed incoherent feed forward loop is shown in Fig. 3-7-2. We newly developed rm/lac hybrid promoter, which is activated by CI and repressed by LacI (Fig. 3-7-3). We plan to ligate a hormone synthase part downstream of this hybrid promoter. Our mathematical model (Fig. 3-7-4) shows what the temporal pattern for plant hormone production should achieve (details about this model can be found here). While rm/lac hybrid promoter activation by CI is a single-step reaction, repression by LacI is a two-step reaction. Thus, the activation of rm/lac hybrid promoter is faster than its repression. This time lag between activation and repression is important for generating a temporal pattern of plant hormone production.
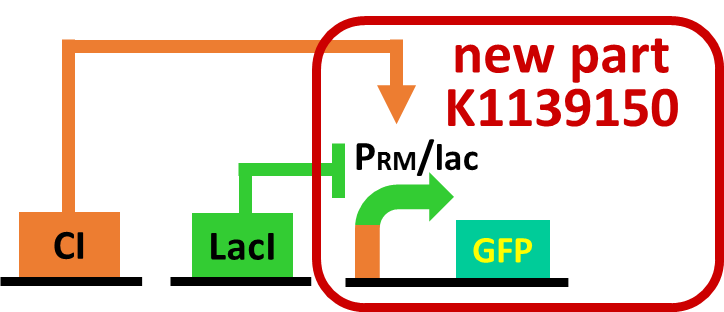
As a first step to achieve this incoherent feed forward loop, we constructed the circuit shown in Fig. 3-7-5 to confirm that our new rm/lac hybrid promoter actually works. We set GFP as a reporter of rm/lac hybrid promoter and introduced the part into E. coli . First, we verified that our rm/lac hybrid promoter is activated by CI with the following assay.
2. Materials and Methods
2-1. Construction
-pSB6A1-Ptet-GFP (N99)…positive control
-pSB6A1-promoterless-GFP (N99)…negative control
-pSB6A1-Prm/lac-GFP (N99)…sample with CI*
-pSB6A1--Prm/lac-GFP (JM2.300)…sample without CI
* This N99 expresses CI from its genome constitutively.
2-2. Assay protocol
- Prepare overnight culture of each cell at 37°C for 12 hours.
- Take 30 microL of the overnight cultures into LB (3 mL) containing antibiotics (Amp 50 microg/mL) and 1 mM of IPTG* (→fresh culture)
*We added IPTG in order to make sure to repress the expression of LacI derived from E. coli genome.
- After 4 hours of induction, flow cytometer measurements for GFP expression.
*We added IPTG in order to make sure to repress the expression of LacI derived from E. coli genome.
3. Results
Fig. 3-7-7 shows the fluorescence intensity detected by flow cytometer. Fig. 3-7-8 is the extracted data which shows the comparison of N99 (IPTG+, with constitutive CI) and JM2.300 (IPTG+, without CI).
4. Discussion
N99 cells (CI+) showed higher fluorescence intensity than JM2.300 cells (CI-). From this experiment, we assume that our rm/lac hybrid promoter is actually activated by CI. We are now confirming that our rm/lac hybrid promoter is not only activated by CI but also repressed by LacI.
 "
"