Team:Evry/ChemicalTools
From 2013.igem.org
(Difference between revisions)
| Line 17: | Line 17: | ||
</ul> | </ul> | ||
The production of each one of those enzymes can thus become a rate-limiting-step, when it comes to mass enterobactin production.</p> | The production of each one of those enzymes can thus become a rate-limiting-step, when it comes to mass enterobactin production.</p> | ||
| - | <img src="https://static.igem.org/mediawiki/2013/1/1d/Entero_pathway.jpg"/> | + | <img src="https://static.igem.org/mediawiki/2013/1/1d/Entero_pathway.jpg"/><br/> |
| - | For now, we consider each one of these steps as a simple chemical reaction: | + | <p> |
| - | + | For now, we consider each one of these steps as a simple chemical reaction:<br/> | |
| - | + | <img src="https://static.igem.org/mediawiki/2013/c/c8/Spe.jpg"/><br/> | |
| - | + | We are using the enzymatic kinetic model of Michaelis-Menten, which divides each reaction into two consecutives steps:<br/> | |
| - | We are using the enzymatic kinetic model of Michaelis-Menten, which divides each reaction into two consecutives steps: | + | <img src="https://static.igem.org/mediawiki/2013/7/70/Esesep.jpg"/><br/> |
| - | + | The speed of the reaction is calculated as below:<br/> | |
| - | + | <img src="https://static.igem.org/mediawiki/2013/b/bd/Vit_reac.jpg"/><br/> | |
| - | + | The steady state approximation gives us:<br/> | |
| - | The speed of the reaction is calculated as below: | + | <img src="https://static.igem.org/mediawiki/2013/3/39/Calcul_vit_reac.jpg"/><br/> |
| - | + | And thus,<br/> | |
| - | + | <img src="https://static.igem.org/mediawiki/2013/5/5e/Resultat_vit_reac.jpg"/><br/> | |
| - | + | where <img src="https://static.igem.org/mediawiki/2013/6/68/Km.jpg"/> and <img src="https://static.igem.org/mediawiki/2013/6/69/Kcat.jpg"/> are classic kinetic parameters. | |
| - | The steady state approximation gives us: | + | </p> |
| - | + | <h2>Conclusion:</h2> | |
| - | + | <p> | |
| - | + | For simple enzymatic reactions (one reagent, one product and one enzyme) with the steady state approximation, we can directly apply the formula above-written.</p> | |
| - | And thus, | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | where | + | |
| - | + | ||
| - | Conclusion: | + | |
| - | + | ||
| - | For simple enzymatic reactions (one reagent, one product and one enzyme) with the steady state approximation, we can directly apply the formula above-written. | + | |
</div> | </div> | ||
Revision as of 08:00, 2 October 2013
Chemical tools
There are 6 enzyms involved in the natural process of the enterobactin production:
- EntA :
- EntB :
- EntC :
- EntD :
- EntE :
- EntF :

For now, we consider each one of these steps as a simple chemical reaction:

We are using the enzymatic kinetic model of Michaelis-Menten, which divides each reaction into two consecutives steps:

The speed of the reaction is calculated as below:
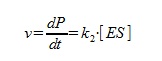
The steady state approximation gives us:

And thus,

where  and
and  are classic kinetic parameters.
are classic kinetic parameters.
Conclusion:
For simple enzymatic reactions (one reagent, one product and one enzyme) with the steady state approximation, we can directly apply the formula above-written.
 "
"













