Team:TU-Munich/Modeling/Protein Predictions
From 2013.igem.org
(→Conclusion:) |
(→Conclusion:) |
||
| Line 123: | Line 123: | ||
|19% | |19% | ||
|0.318 | |0.318 | ||
| - | |[[File: | + | |[[File:TUM13_small_EreBx.png|85px]] |
|- | |- | ||
|Spycatcher | |Spycatcher | ||
Revision as of 21:31, 19 October 2013
Prediction of Protein Structures and Functions
Structural properties of effector proteins are often important for their function, so it is advantageous to know about them. It is for example necessary to know whether termini are accessible for protein fusion or whether the protein is only functional in a multimeric fold. For this reason a structure based search was performed in the [http://www.rcsb.org/pdb/home/home.do protein data bank]. As the number of identified structures is still limited, it is a promising attempt to look for homologous proteins whose crystal structures have been determined.
Analysis of Receptor Sequences – Choosing the right template
For several purposes of our project, we needed a synthetic receptor enabling us to integrate proteins into the membrane in the desired orientation, i.e. to express protein-domains on the intracellular or extracellular side of the cell membrane. We investigated several different plant-receptors from the well characterized dicotyledon Arabidopsis thaliana and the moss Physcomitrella patens, our chassis. The receptors from Arabidopsis thaliana have the advantage that their transgenic expression has successfully been demonstrated http://www.pnas.org/content/88/23/10806.full.pdf Quail et al., 1991 whereas the receptors from Physcomitrella patens bear less risk that they do not work in the evolutionary far distant moss http://www.plant-biotech.net/paper/Reski_1998_BotActa-111_1_scan.pdf Reski, 1998
Due to the fact that there were many different available receptors, which we could use as a template for our synthetic receptor, we used bioinformatical methods to evaluate the suitability of these receptors. The following three examples ERF, FLS2 and SERK shown in table 1 resulted from this equation.
| Receptor | Organism | Length (aa) | Sequence reference | Literature reference |
|---|---|---|---|---|
| ERF | A. thaliana | 1031 | [http://www.ncbi.nlm.nih.gov/protein/NP_197548.1 NP_197548.1] | |
| FLS2 | A. thaliana | 1173 | [http://www.ncbi.nlm.nih.gov/protein/NP_199445.1 NP_199445.1] | |
| SERK | P. patens | 625 | [http://www.ncbi.nlm.nih.gov/protein/XP_001759122.1 XP_001759122.1] | [http://www.freidok.uni-freiburg.de/volltexte/5390/pdf/Lienhart_Dissertation_2008.pdf Lienhart, 2007] |
Prediction of Signal Peptides
Introduction
A first analysis was performed to identify signal-peptides, which are bound by the cellular signal recognition particle and lead to the translocation of the bound polypeptide into the endoplasmic reticulum. Afterwards the signal peptide is cleaved by a signal peptide peptidase at a specific site. The analysis for signal peptides was done by using the [http://www.cbs.dtu.dk/services/SignalP SignalP 4.1 Server].
Results
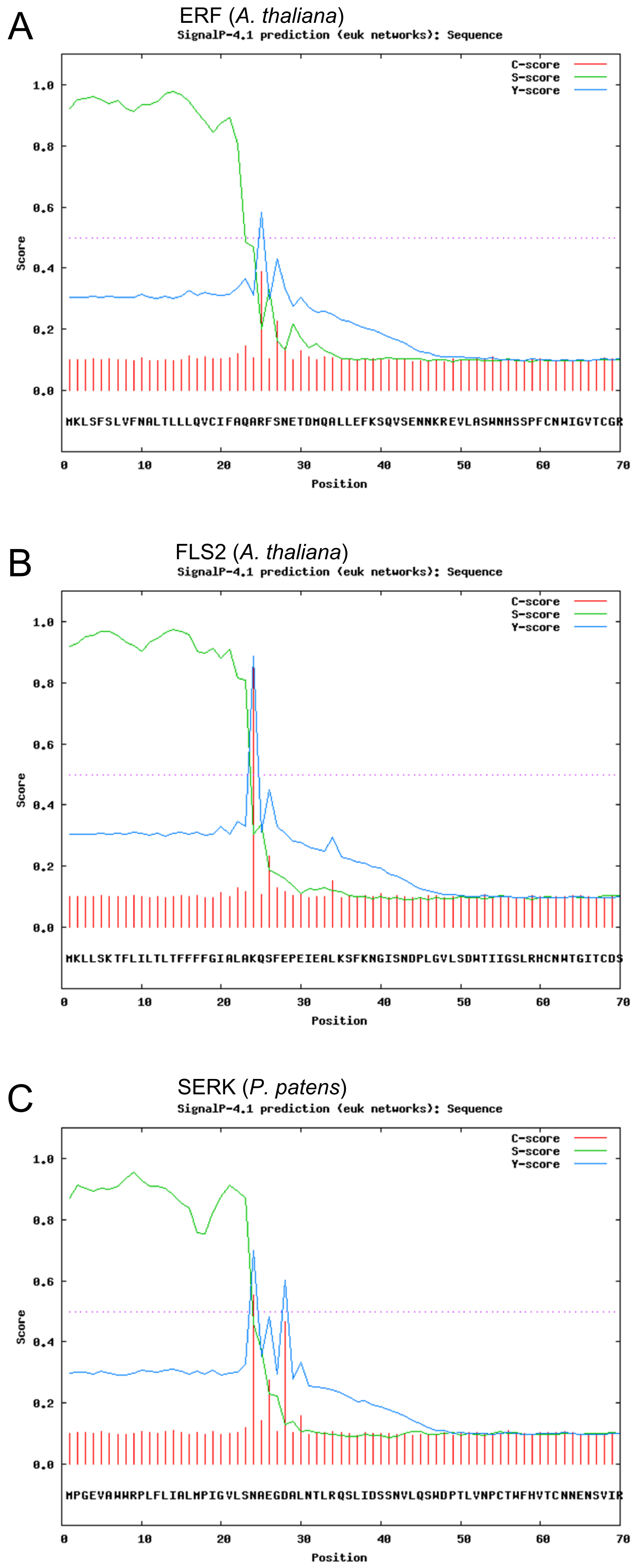
The program was run for different receptors and will be illustrated for the three examples mentioned above (see fig. 1).
The figure shows the N-terminal sequence of the receptors, together with three scores:
(1) The C-Score (raw cleavage site score) in red.
(2) The S-Score (signal peptide score) in green.
(3) The Y-Score (combined cleavage site score) in blue.
The C-Score shows the most probable cleavage site identified by the peptidase. It was possible to identify the most probable cleavage site for all shown receptors with ambiguous cleavage sites for the SERK-receptor. The amino acid with the highest C-score is predicted to be the first amino acid of the primary structure of the cleaved receptor.
The S-Score was developed to identify amino acid sequences which appear in a signal peptide and others that belong to the matured receptor. The course of this parameter is high for the first 23-28 amino acids of all receptors, identifying these residues as signal peptides. The amino acid residue, which lies at the greatest decrease of the S-Score, is the predicted border between the N-terminal signal peptide and the receptor.
The Y-Score results from the geometrical structure of the protein and the previously determined scoring parameters. It illustrates that the two first parameters show a good fit for the identification of the signal peptide in all three indicated receptors.
Discussion
Summarizing these parameters, it can be concluded that all three pictured receptors seem to contain a sequence acting as a signal peptide. For many of the predicted receptors in the genome of Physcomitrella patens the prediction did not yield a positive result. With respect to the signal peptide, all mentioned receptors would be suitable as a template for our synthetic receptor. The predicted data show that the SERK-Receptor is favorable for our application, because its signal peptide is statistically the best recognized one and bears the smallest risk of failure.
Prediction of Transmembrane Regions
Introduction
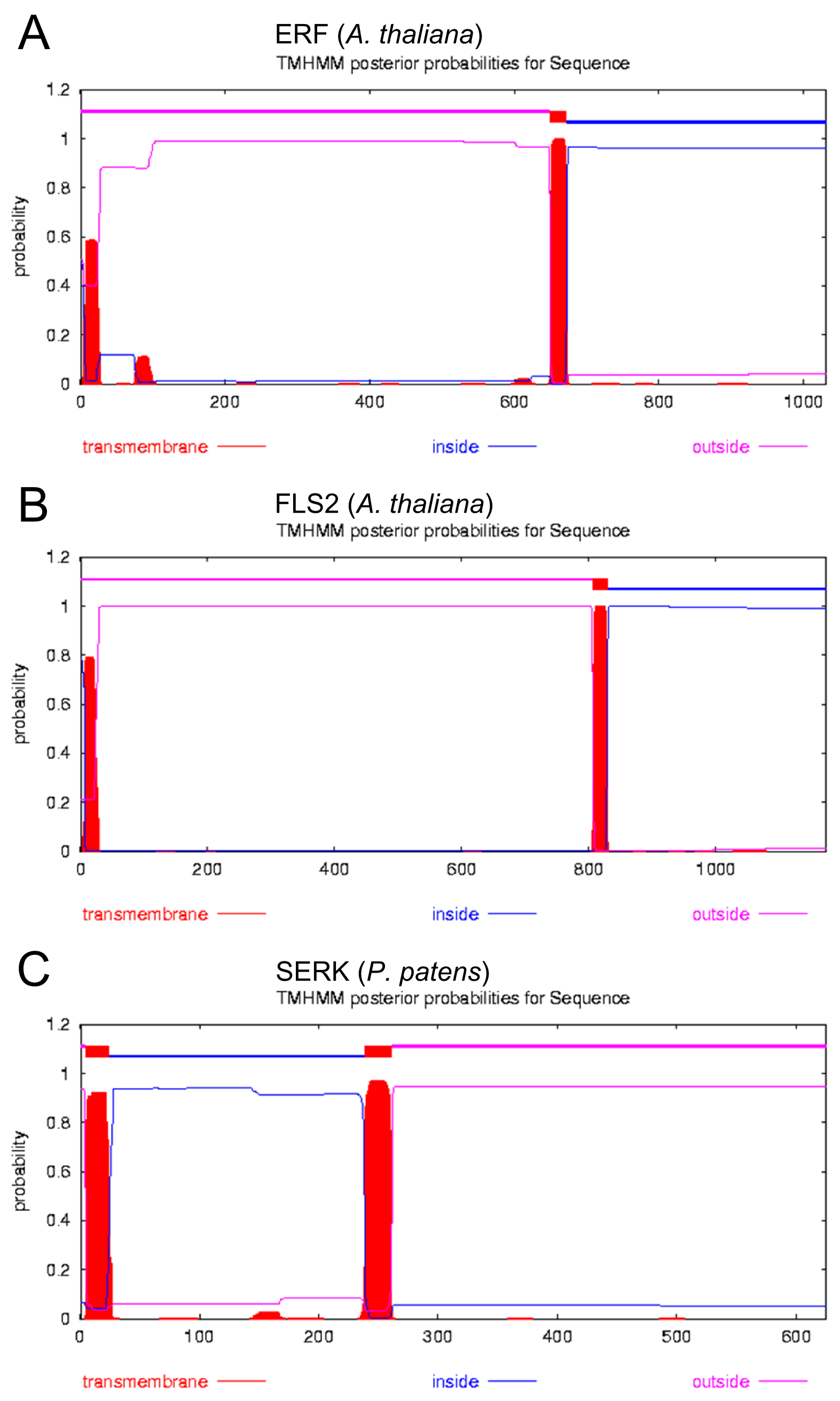
Additional to the identification of the signal peptide, it was very important to identify transmembrane regions within the receptors, because we wanted to use a type I receptor as a template that contains a N-terminal extracellular domain, a transmembrane domain and a C-terminal intracellular domain (see our localization page). To analyze this issue, the prediction tool [http://www.cbs.dtu.dk/services/TMHMM TMHMM] was used for several different receptors. Again the most suitable receptors were ERK, FLS2 and SERK.
Results
The analysis yields a signal peptide and a single transmembrane domain for all three depicted receptors (see fig. 2). The estimated reliability of the predictions was equally good for all examined receptors, whereas the signal peptide was most reliably predicted for the SERK receptor.
Discussion
Focussing on the membrane topology point of view, all the investigated receptors would be suitable blue prints for our synthetic receptor. As the SERK-Receptor yields the best prediction, it was chosen as the favorable template. Another reason to choose the SERK-Receptor was that it is derived from Physcomitrella patens. The only problem, concerning this prediction, is that the N-terminus of this receptor is predicted to be extracellular. The falsification of this prediction was simple, because the SERK receptor contains a C-terminal kinase-domain, which is known to be involved in signal transduction.
Choice of the SERK Receptor
Finally we decided to use the SERK receptor as a template to generate our synthetic receptor. The final receptor was designed in RFC[25] standard, which allows in frame protein fusions. The final constructs were designed containing the SERK signal peptide ([http://parts.igem.org/Part:BBa_K1159303 BBa_K1159303]), an extracellularly located effector protein, the transmembrane domain of the SERK receptor ([http://parts.igem.org/Part:BBa_K1159305 BBa_K1159305]), a short linker and a GFP, to investigate the cellular localization of our receptor with the aid of fluorescence microscopy.
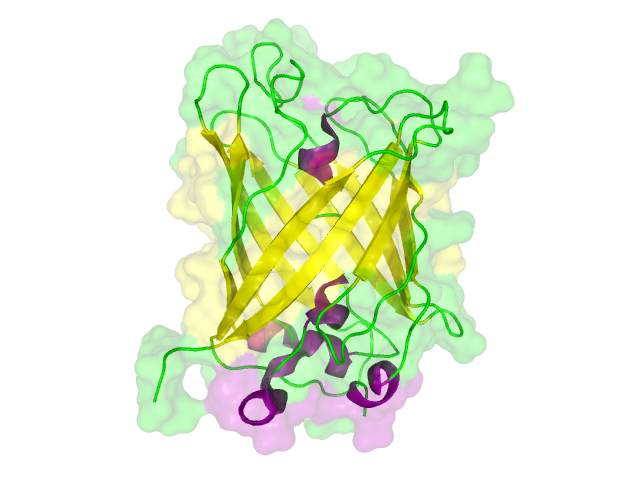
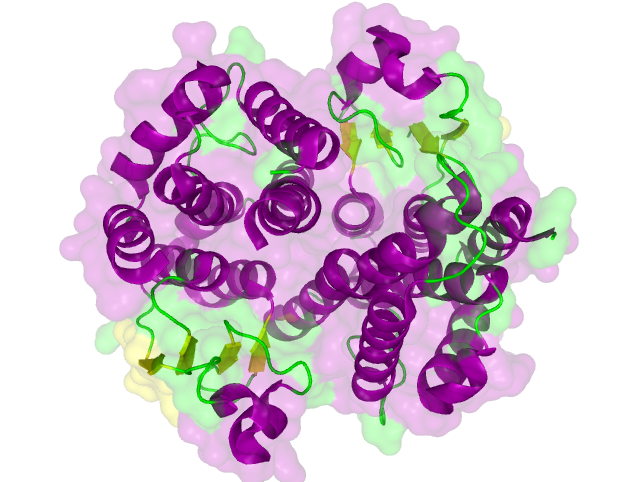
Searching for Homologous Structures using HHpred
Aim:
Approach:
Results:
Conclusion:
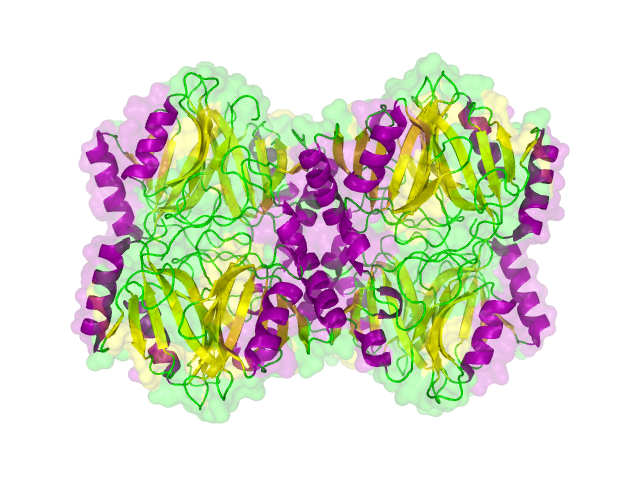
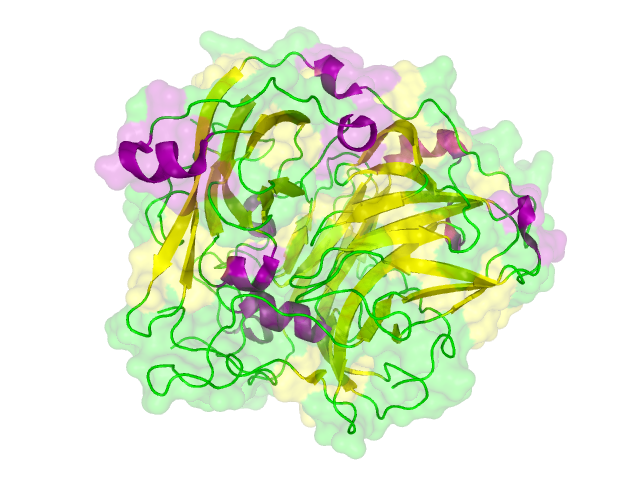
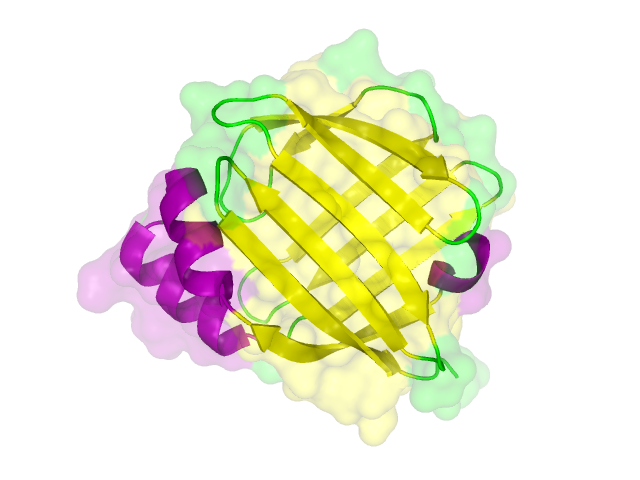
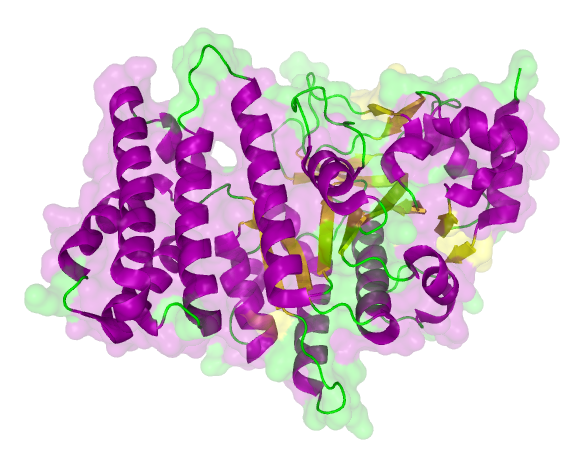
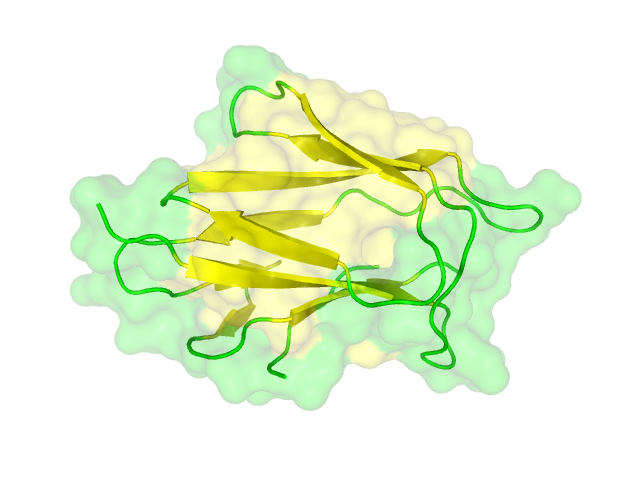
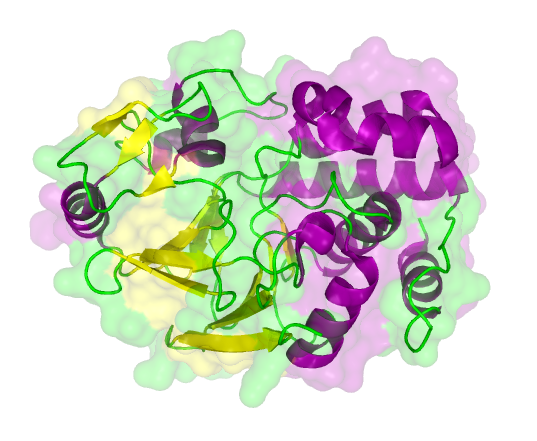
The search for homologous structures was performed by using the freely accessible web server [http://toolkit.tuebingen.mpg.de/hhpred HHpred] http://www.ncbi.nlm.nih.gov/pubmed/15980461 Söding et al., 2005. The amino acid sequences for the BioBricks were translated into amino acid sequences using the AutoAnnotator and was then inserted into the the search field. The results for all proteins investigated in our project are shown in table 2.
Results
The homology search showed that some of our effector proteins have very closely related proteins with a known structure. For example there are very similar protein structures available for the SypCatcher, PP1 and GFP each with identities of over 90%. Some other effector proteins such as XylE, Laccase or the DDT Dehydrochlorinase have less homologous proteins, whose structures still give good hints on structural questions. However there are also effectors where only badly matched structures are known, which can only be used as a very rough indication of the fold. The NanoLuc luciferase, which is a highly engineered protein derived from shrimps and was only published this year, is an example of a protein with no known structural homologue.
The structures obtained here were used to design our experiments. A homology modeling for the Laccase was performed to determine whether it contains disulphide bridges. The resulting homologous structures were used as illustrations, as explained in one of our How-Tos about animated GIFs.
References:
http://www.ncbi.nlm.nih.gov/pubmed/15980461 Söding et al., 2005 Söding J, Biegert A, Lupas AN. (2005). The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005 Jul 1;33(Web Server issue):W244-8.
http://www.plant-biotech.net/paper/Reski_1998_BotActa-111_1_scan.pdf Reski, 1998 Reski, R. (1998). Development, Genetics and Molecular Biology of Mosses. Bot. Acta, 111:1-15.
http://www.pnas.org/content/88/23/10806.full.pdf Quail et al., 1991 MARGARET T. Boylan, M.T. and Quail, P.H. (1991). PhytochromeA overexpression inhibits hypocotyl elongation in transgenic Arabidopsis. Proc. Natl. Acad. Sci. 88:10806-10810.
http://www.freidok.uni-freiburg.de/volltexte/5390/pdf/Lienhart_Dissertation_2008.pdf Lienhart, 2007 Lienhart, O. (2007). Untersuchungen zu einem Somatic-Embryogenesis-Receptor-like-Kinase-Homolog in Physcomitrella patens (Hedw.) B.S.G. PhD-thesis at Freiburg University
 "
"




AutoAnnotator:
Follow us:
Address:
iGEM Team TU-Munich
Emil-Erlenmeyer-Forum 5
85354 Freising, Germany
Email: igem@wzw.tum.de
Phone: +49 8161 71-4351