Team:NTU-Taida/Project/Circuits
From 2013.igem.org
Contents |
Introduction
To reach our goal – real-time detection and identification of drug-resistance bacterial strains, we have proposed two ways to fulfill it. First, enhance the ability to sense quorum sensing molecules at low concentration. In other words, detect bacteria earlier, and shorten the time on culturing. Second, accelerating the speed of downstream response after our QS array is turned on, thus we could get the result as soon as possible.
We have designed two kinds of circuits based on the ways mentioned early. One contains a positive-feedback loop, while the other works on a negative-regulation mechanism. We also made basic circuits without any feedback, as the comparison of our designs.
It was said by team Northwestern, 2011 that if the reporter region was put inferior in the circuit, the response would not be obvious enough to detect. However, theoretically speaking, this seems unreasonable. Due to curiosity, most of our circuits were made in two forms, with the reporter putting either forward or afterward, in order to reproduce the phenomenon and try to explain it.
We’ll make detailed description of our designs below.
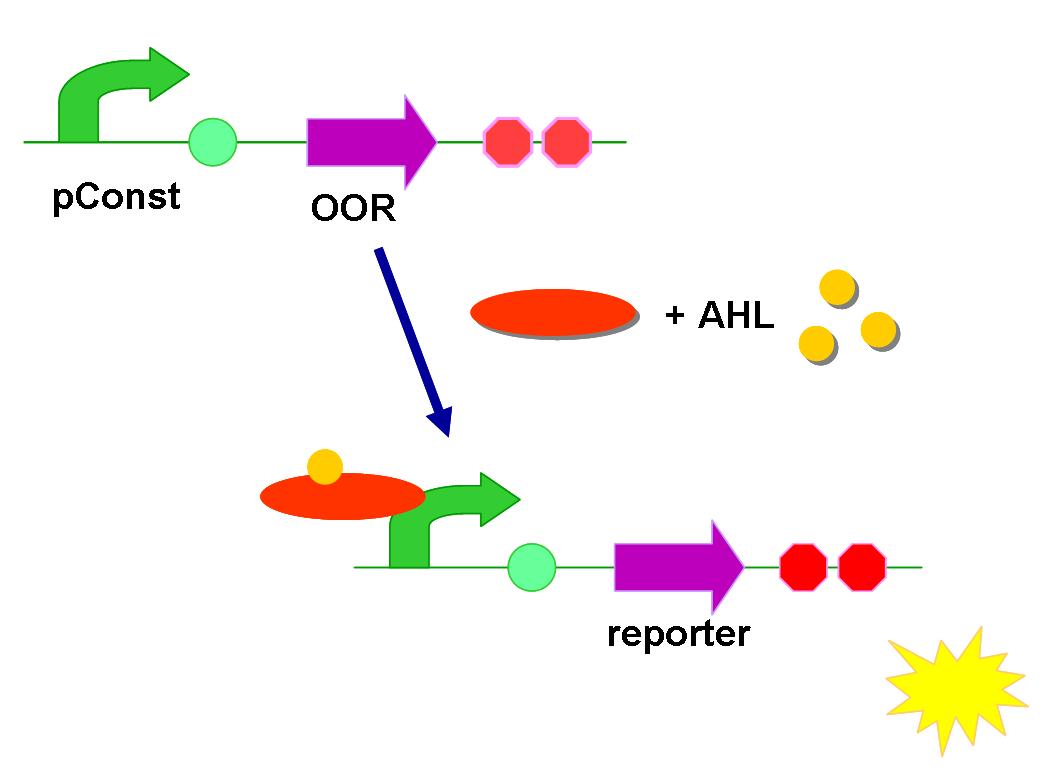
Fundamental circuit
Similar to Bba_K575024 and Bba_K575033 from team Northwestern, 2011, the basic design is a double-level devise. It contains a constitutive expression quorum sensing receptor, and a reporter regulated by QS regulated promoter.
Once the specific AHL molecule binds on the receptor, the complex will activate its corresponding promoter. At this time, fluorescent protein (reporter) would express. It is a simple input-output circuit.
Two kinds of arrangement of biobricks were made. They truly had difference! It was shown in Result.
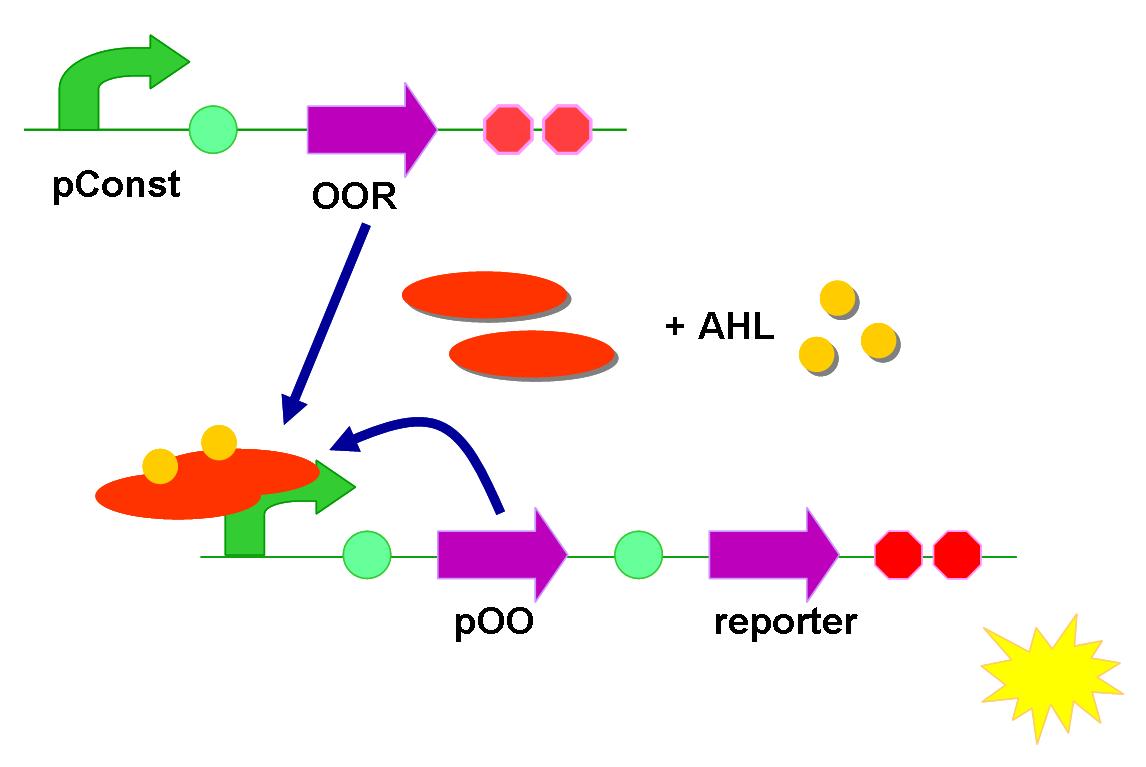
Positive feedback
Based on the fundamental one, we involved a positive-feedback loop in this time. A QS receptor gene was added in between the QS promoter and the reporter.
After activation by AHL, more QS receptor would be produced. According to Le Chatelier's principle with the equation
$$ AHL + receptor -> AHL-receptor complex $$
If we have more receptors (on the left side), more complexes (the right side) will be output. More complex means that the promoter will be activated more completely, leading to stronger fluorescent signal even though AHL are still at low concentration.
The same problem about the position of reporter might exist in this circuit. Again, we let the region under the regulation of QS promoter (contains reporter) either in front of or behind the constitutive-expressing region.
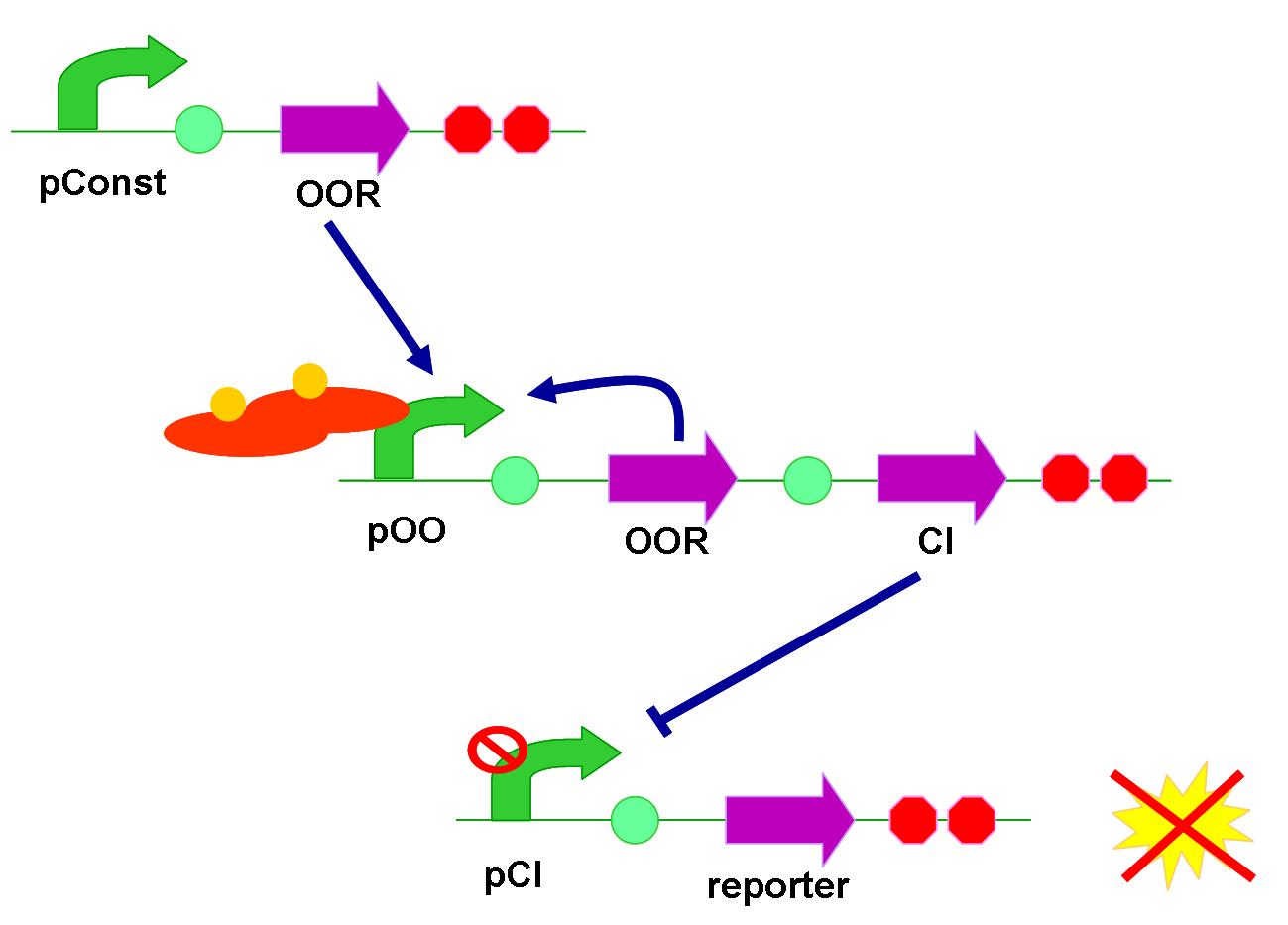
Negative regulation
The purpose of positive feedback design is for higher sensitivity to AHL. But how about the reaction time after turning on the circuit? Transcription and translation are two indispensable steps for producing a fluorescent protein (reporter), yet sometimes the procedure lasts too long. Referring to a paper in 2005, the negative regulation design involves CI gene in the circuit, using a pre-produce mechanism rather than post-produce to reduce reaction time. And still, the positive feedback of QS receptor is reserved in this circuit.
At normal time, CI promoter (pCI) can be viewed as a constitutive promoter, so fluorescent proteins are continuously produced. When our array detects target AHL molecules, QS promoter is activated, starting produce CI. CI is the repressor of pCI, making the production of fluorescent protein stopped. In this situation, the total signal would slowly weaken since fluorescent proteins are degraded. Besides, bacteria keep on multiplying their population. The denominator (cell number) increases while the numerator (fluorescent protein) doesn’t; as a consequence, the fluorescent signal in a single cell gradually drops.
The negative-regulation circuit has three levels. With similar principle, we assembled them in two ways. One followed the regulative orders, and the other put reporter region foremost.
David Karig, Ron Weiss.. Signal-Amplifying Genetic Circuit Enables In Vivo Observation of Weak Promoter Activation in the Rhl Quorum Sensing System., Biotechnol Bioeng. 2005 Mar 20;89(6):709-18..
Reference
 "
"