Team:NTU-Taida/Result/AHL Concentration
From 2013.igem.org
Contents |
Functional results
We have designed three types of quorum sensing biosensors, one with a receptor and reporter, another with a positive feedback and the other with a CI-pCI inhibitory circuit(negative regulation) (see circuits for information ). We tested the functions of these biosensors quantitatively and qualitatively with the following experiments. We hope to compare the efficiency between these 3 types of circuits and hope to optimize the design of quorum-sensing-based biosensors.
Qualitative experiments
To test whether these biosensors are sensitive to acyl homoserine lactones (AHLs), we cultured transformed DH5α E. Coli under 37∘C overnight(with biobrick BBa_K575024). We sprayed 0.1 MC-4 AHL over the E. Coli and waited for 4 hours to check results.
Furthermore, we tested the protein expression of different types of biosensors. We compared
- DH5α
- Rhl with positive feedback with constitutive promoter placed in front
- [http://parts.igem.org/Part:BBa_K575033 K575033](link)
- [http://parts.igem.org/Part:BBa_K575024 K575024](link)
We use SDS-PAGE to check the expression of protein stained by coomassie blue under the existence of AHLs.
The results showed that K575024 protein expression increased significantly around 25-30 kD, which is about the size of GFPmut3 (fluorescence reporter).
Quantitative experiments
We tested the functions of different types of circuits with ELISA plate reader and flow cytometry.
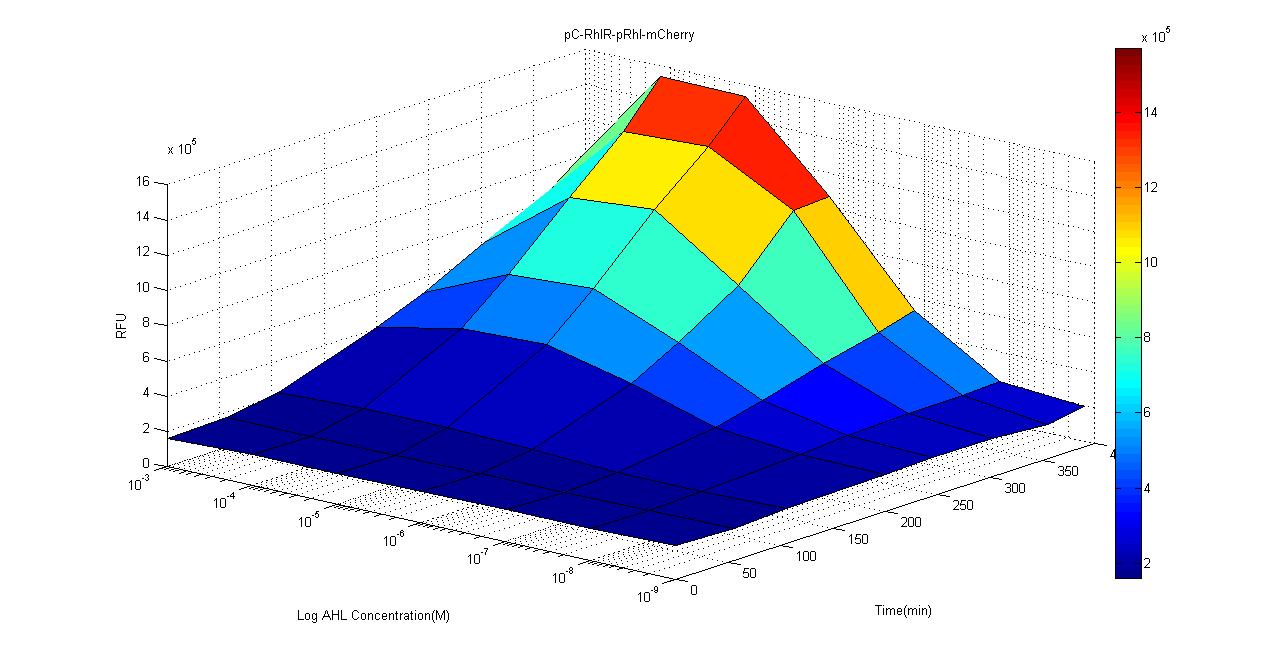
Rhl-mCherry without positive feedback
- pConst-RBS-RhlR-tt-pRhl-RBS-mCherry-tt
We used ELISA plate reader to test the mCherry fluorescent expression under different concentration of C-4 AHLs and data are recorded every hour for 4 hours. The results are as follows.Compared to the control group, intensity of mCherry signal is significantly stronger when concentration is over 10-7M. We suspect that due to the high percentage of acetyl acetate which is used to suspend the AHL, in the 10-3 group, the acetyl acetate may affect the growth and fluorescence expression of our biosensor. According to studies, the concentration of AHL is around μM level, which is around the sensitivity limit of our biosensor, and we believe that this gives us a good reason to use this circuit as a clinical biosensor.
-
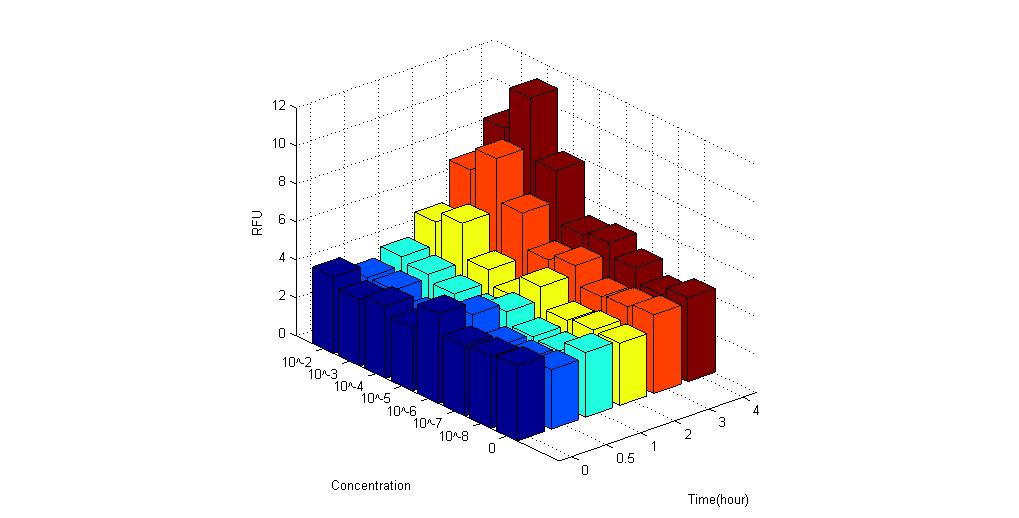
- Pc-RBS-RhlR-tt-pRhl-RBS-RhlR-RBS-GFPmut3-tt
- pRhl-RBS-RhlR-RBS-GFP-tt-Pc-RBS-RhlR-tt
- The 2 constructs above are similar except different order of biobrick parts. 1 with constitutive promoter placed in front while the other with the constitutive promoter placed in the middle.
We used ELISA plate reader to test the GFPmut3 fluorescence expression under different concentration of C-4 AHLs. The results are as follows.
It is obvious that these biosensors express fluorescence when placed under AHL compared to control group. Therefore, the results proved to be positive.
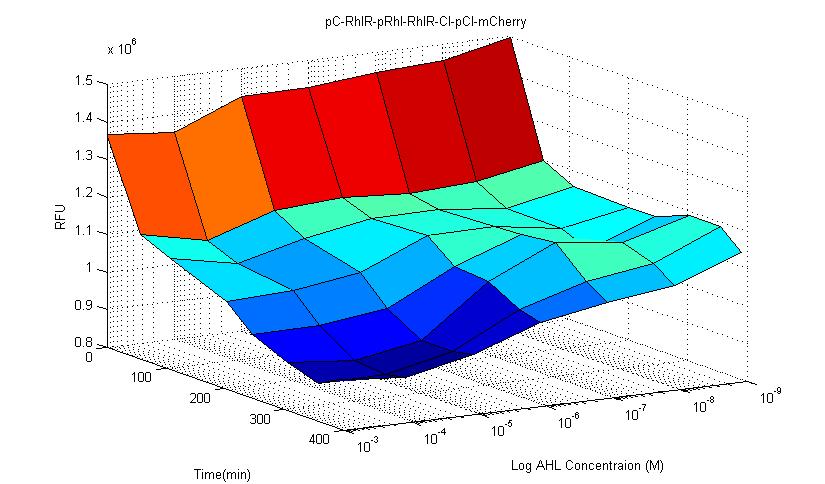
- Pc-RBS-RhlR-tt-pRhl-RBS-RhlR-RBS-CI-tt-pCI-RBS-mCherry-tt
In this circuit, fluorescent protein mCherry and receptor RhlR are constitutively expressed. When RhlR combines with C-4 AHL, it binds to its promoter sequence (pRhl) and induces the production of more Rhl receptor and inhibitory protein CI. CI then binds to its inhibitory sequence pCI and inhibit mCherry expression. Therefore, if this circuit is successful, fluorescence expression should be significantly lower than control group. The results showed that after 4 hours it is obvious that the fluorescence is lower than control group.In our experiment, we observed that in this type of circuit, signal noise is reduced compared to other types of circuits. This circuit has better stability compared to other constructed biosensors.
Rhl-GFP with positive feedback
Rhl-mCherry with CI-pCI circuit
 "
"