Team:ETH Zurich/Experiments 3
From 2013.igem.org
Contents |
Enzyme-substrate reactions
To generate visible output by adding substrates to colonies in Colisweeper, we made use of orthogonal enzyme-substrate reactions. A set of chromogenic substrates was chosen to produce different colors depending on the abundant hydrolases and thereby to uncover the identity of each colony to the player.
The set of enzyme-substrate pairs chosen for the Colisweeper project, and characterizations of their reactions, are described below. Information on other possible substrates that can be used for the enzymes of the Colisweeper reporter system can be found in the reporter system section.
To characterize the hydrolases used in Colisweeper, we conducted substrate tests in liquid cultures as well as on colonies, enzyme kinetics and detection of HIS-tagged protein by Western Blot. Additionally, crosstalk assays and color overlay tests (multiple enzyme-substrate reactions together) have been performed for this project. An overview of chromogenic substrates used can be found in the Materials section.
Expand the boxes to see the characterization of the hydrolases
Acetyl esterase (Aes)
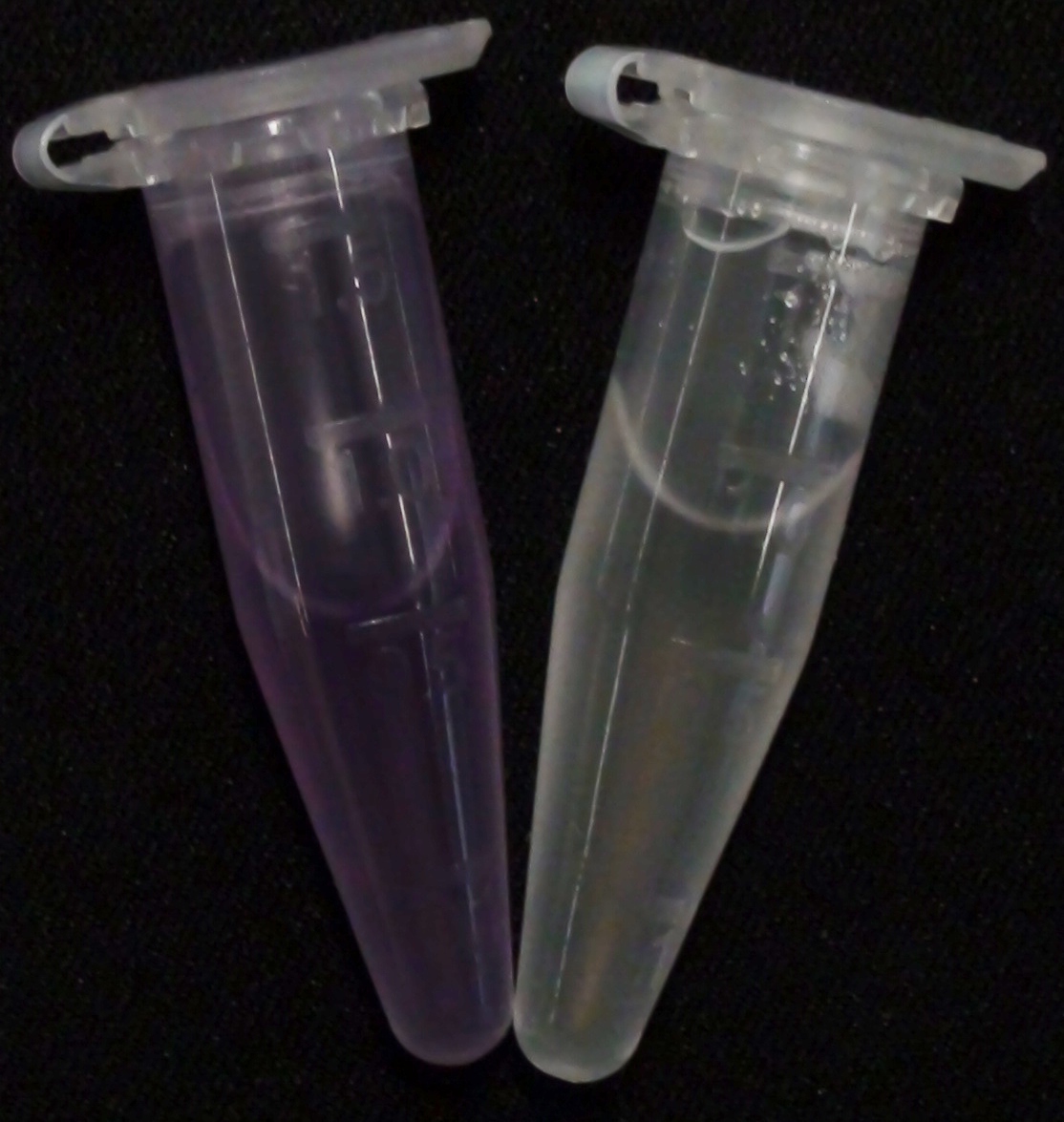
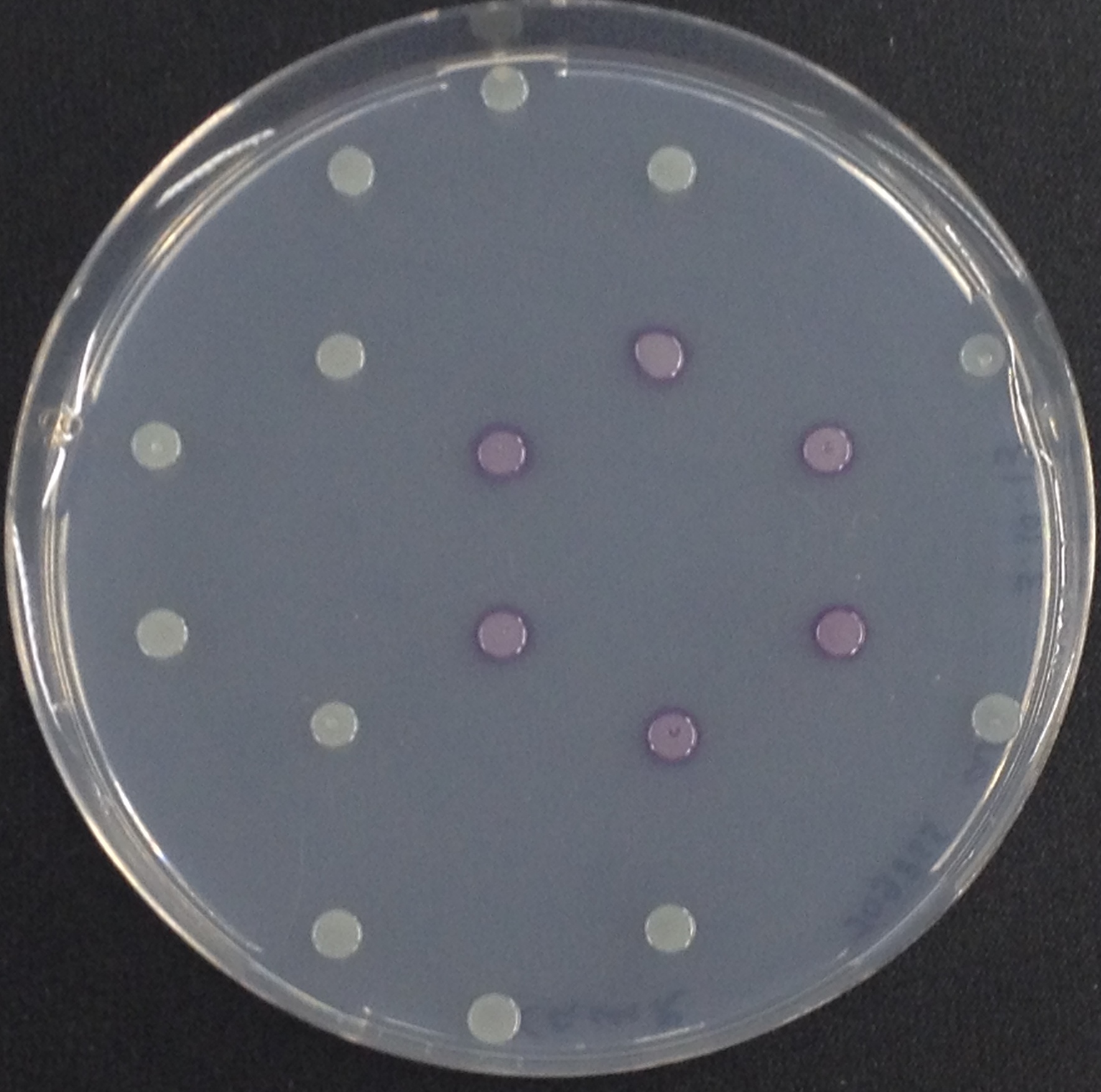
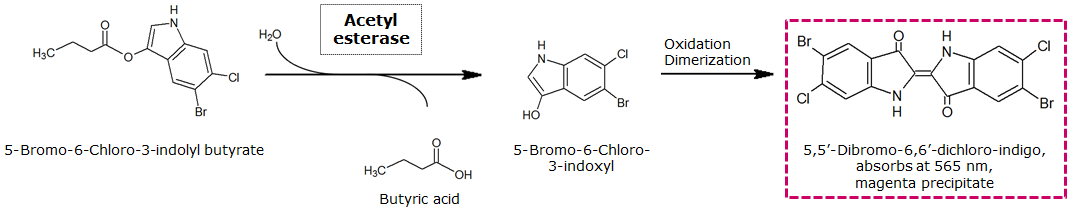
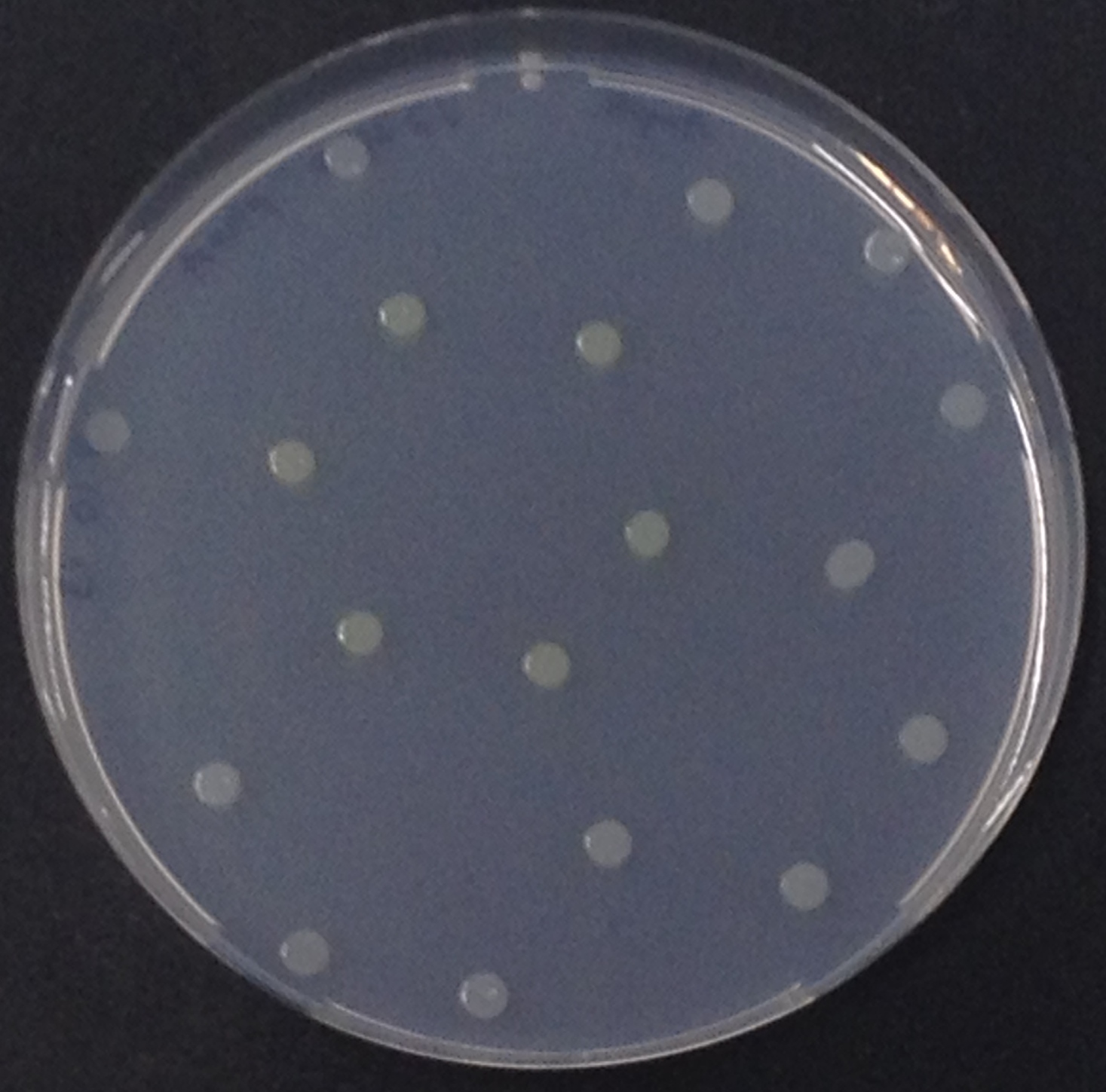
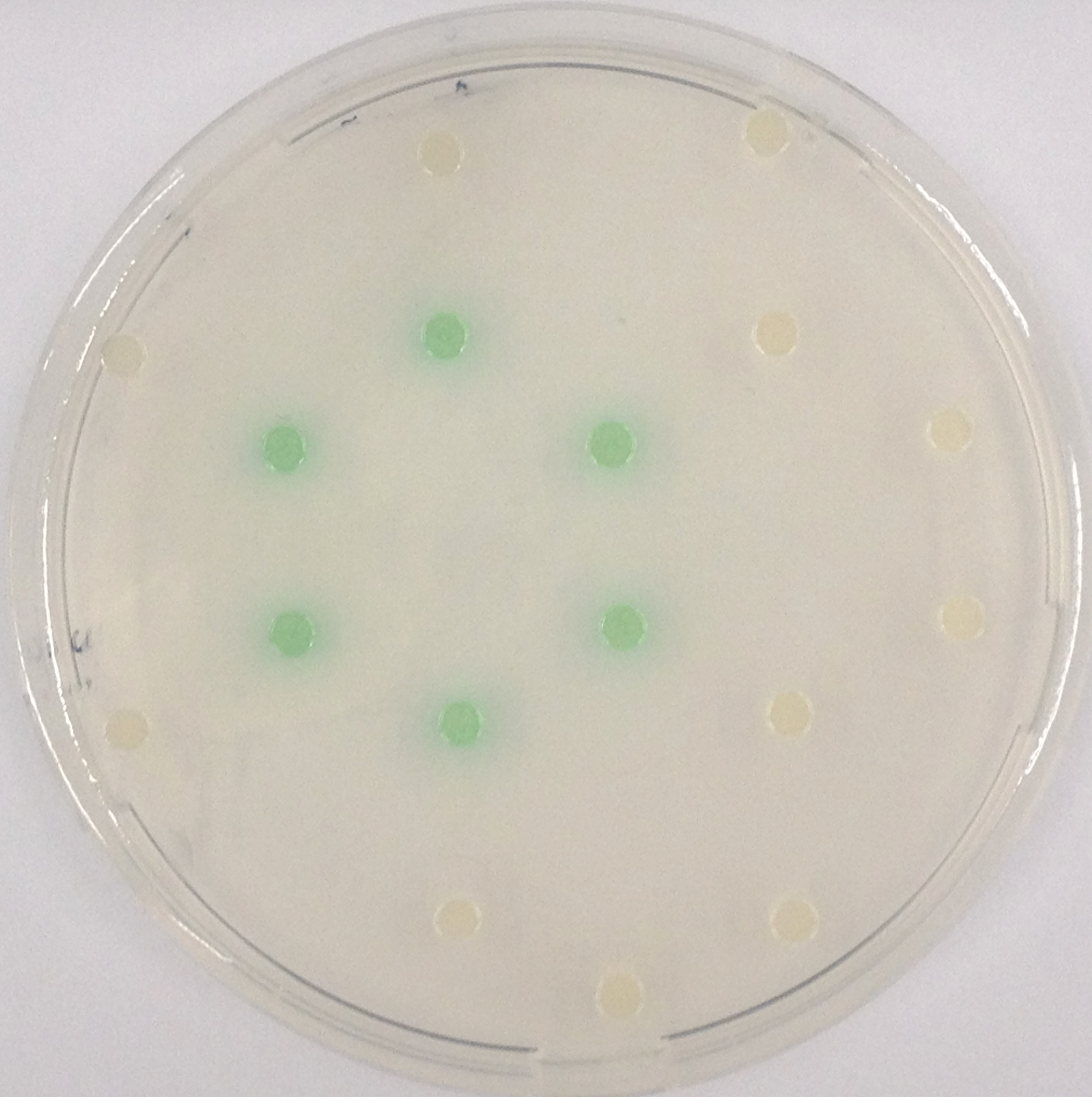
To assess color development after reaction of the enzyme with the chromogenic substrate, a liquid culture of our triple knockout Escherichia coli strain overexpressing Aes was grown until an OD600 of 0.4 - 0.6 was reached before addition of 5-Bromo-6-Chloro-3-indolyl butyrate (magenta butyrate) to a final concentration of 100 µM (see Figure 2). To study the color development in the actual Colisweeper game setup, colonies were plated by pipetting 1.5 µl of the triple knockout Escherichia coli liquid culture (OD600 of 0.4 - 0.6) on an M9 agar plate. Addition of 1.5 µl of 20 mM magenta butyrate onto each colony results in color generation visible after a few minutes at room temperature (see Figure 3).
Enzyme kinetics
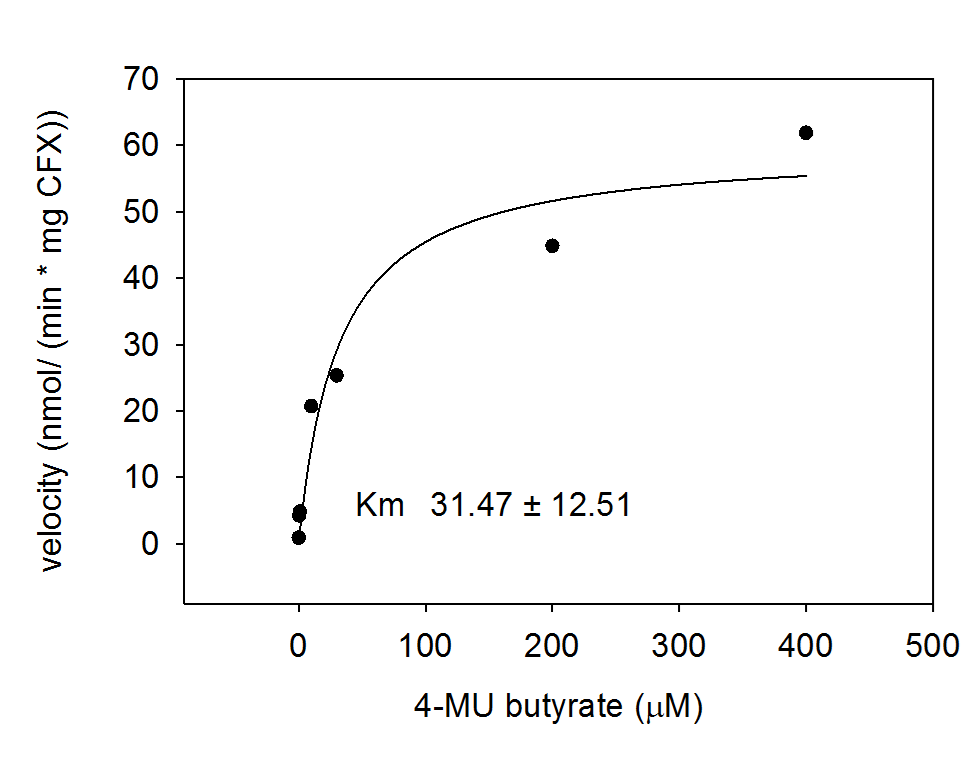
For the kinetics assay, the Escherichia coli lysate overexpressing Aes was tested with the fluorescent substrate 4-MU-butyrate. The picture on the right (Figure 5) was taken with a common single lens reflex camera mounted on a dark hood at λEx 365 nm.
To characterize the enzyme, we conducted Michaelis Menten kinetics to obtain the Km value. Escherichia coli cells were harvested and lysed, and the cell free extract (CFX) was then collected for the fluorometric assay. The properly diluted CFX was measured on a 96 well plate in triplicates per substrate concentration. The plate reader took measurements at λEx 365 nm and λEm 445 nm and determined a Km value of 31.5 ± 12.5 μM. The obtained data was then evaluated and fitted to Michaelis-Menten-Kinetics with SigmaPlot™:
Alkaline phosphatase (PhoA)
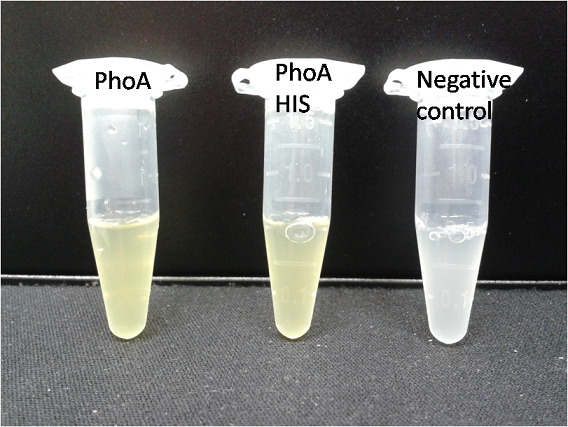
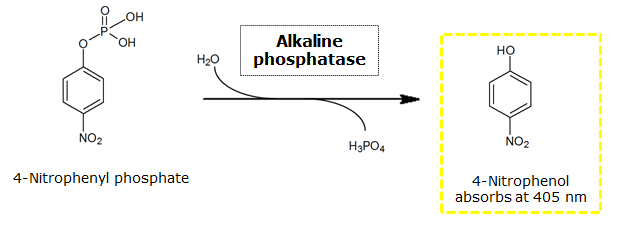
To test the functionality of this PhoA, a liquid culture of Escherichia coli overexpressing this hydrolase was grown until an OD600 of 0.4 - 0.6 was reached before addition of p-nitrophenyl phosphate (pNPP) to a final concentration of 5 mM. This substrate gives rise to yellow color after hydrolysis by PhoA (Figure 8). In the Colisweeper game, substrates are pipetted onto colonies. Figure 10 shows color development on colonies five minutes after addition of 1.5 µl of a 0.5 M pNPP solution.
Characterization of PhoA-HIS tagged protein
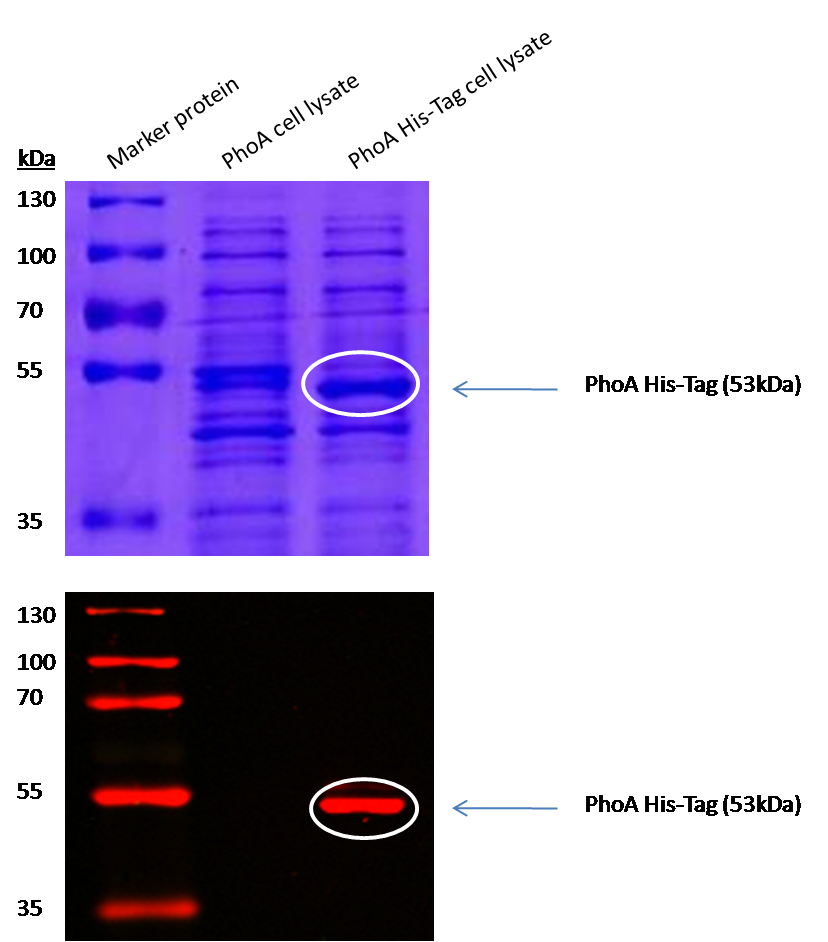
The PhoA and PhoA with HIS-tag were overexpressed in the Escherichia coli BL21 DE3 strain, induced by 5 μM IPTG (iso-propy-β-D-1-thiogalactopyranoside) and finally harvested in order to obtain the cell lysate by lysozyme lysis. This cell lysate of both cultures were analyzed next to each other in two SDS-PAGE gels, one for comassie staining (blue gel in the picture) and one for western blotting (black picture) with Anti-6X His tag® antibody from mouse and a second reporter goat anti mouse antibody with a IRDye 680RD. In the picture we can distinguish the PhoA His (53 kDa) on the western blot as well as on the SDS-PAGE gel (see white circles).
Enzyme kinetics
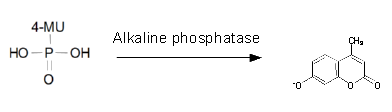
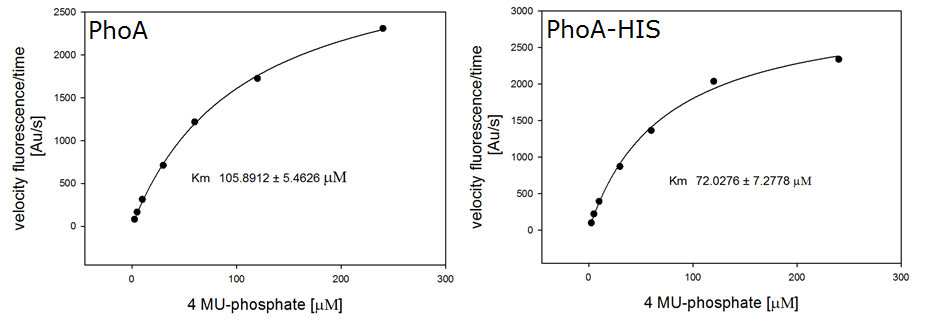
For the kinetics assay, we tested both the Citrobacter phoA and phoA-HIS encoded proteins with the fluorescent substrate 4-MU-phosphate. The image in Figure 12 was taken with a common single lens reflex camera mounted on a dark hood at λEx 365 nm.
To characterize the enzymes, we conducted fluorometric assays to obtain Km values. Escherichia coli were harvested and lysed, and the cell free extract (CFX) was then collected for the fluorometric assay. The properly diluted CFX was measured on a 96 well plate in triplicates per substrate concentration. The plate reader took measurements at λEx 365 nm and λEm 445 nm, determining a Km values of 105.9 ± 5.3 μM for PhoA and 72.0 ± 7.3 μM for Pho-HIS.
The obtained data was evaluated and finally fitted to Michaelis-Menten-Kinetics with SigmaPlot™:
β-Galactosidase (LacZ)
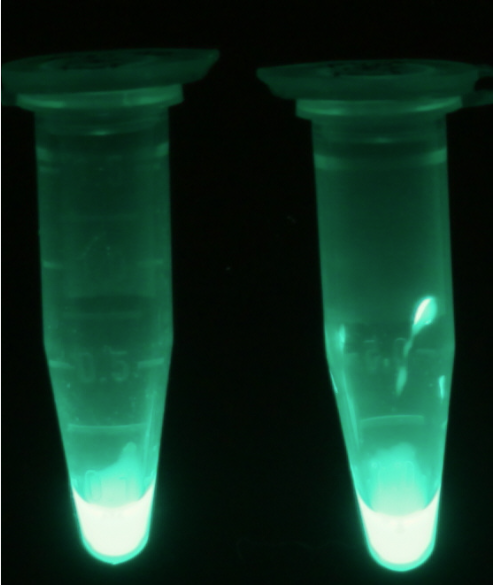
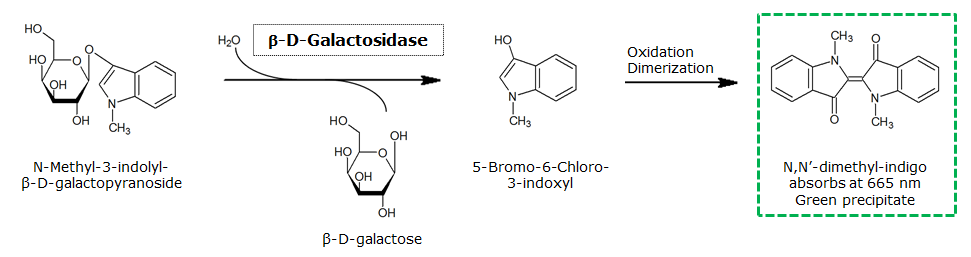
The β-Galactosidase is natively expressed in the triple knockout Escherichia coli strain used in Colisweeper. Its commonly used chromogenic substrate is 5-Bromo-4-Chloro-3-indolyl-β-D-Galactopyranoside (X-Gal), which yields a blue-colored precipitate (Figure 15, middle). In Colisweeper, this protein catalyzes hydrolysis of the flagging substrate, which is N-Methyl-3-indolyl-β-D-Galactopyranoside (Green-β-D-Gal) and produces a green color upon cleavage of the chromophore (Figure 15, left tube). For the color assay in liquid culuture, we used Escherichia coli cultures grown to an OD600 of 0.4 - 0.6, then added the substrates to a final concentration of 50 mM.
β-Glucuronidase (GusA)
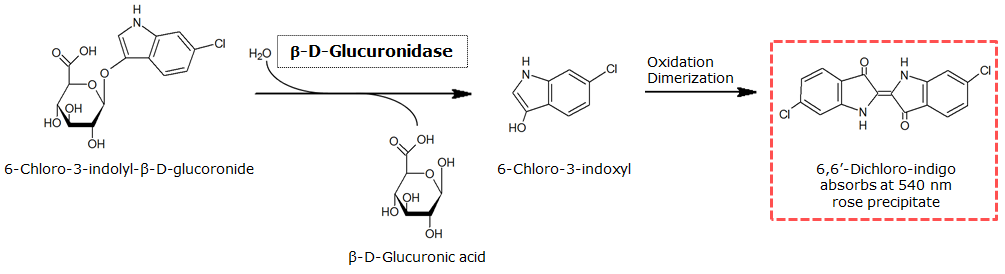
To test the functionality of GusA, a liquid culture of Escherichia coli overexpressing GusA was grown until an OD600 of 0.4 - 0.6 was reached before addition of 6-Chloro-3-indolyl-β-glucuronide (Salmon-Gluc), a substrate for GusA which produces a rose/salmon color after cleavage. The final concentration of the substrate in liquid culture was 1 mM and the conversion of this substrate was done by incubation at 37°C.
Enzyme kinetics
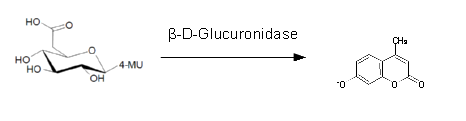
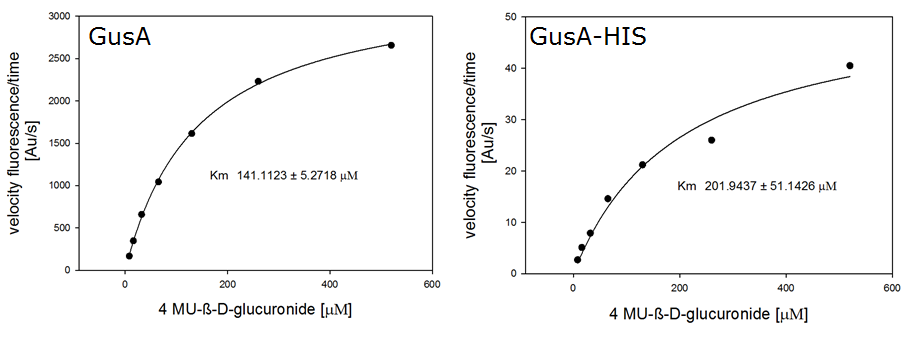
For the kinetics assay, we tested both gusA and gusA-HIS encoded proteins with the fluorescent substrate 4-MU-β-D-Glucuronide. The picture on the right (Figure 20) was taken with a common single lens reflex camera mounted on a dark hood at λEx 365 nm.
For the enzyme kinetics of GusA, we conducted fluorometric assays to obtain the Km value. Escherichia coli overexpressing GusA were harvested and lysed, and the cell free extract (CFX) was then collected for the fluorometric assay. The properly diluted CFX was measured on a 96 well plate in triplicates per substrate concentration. A plate reader took measurements at λEx 365 nm and λEm 445 nm, and determined a Km value of 141.1 ± 5.3 μM for GusA, and 201.9 ± 51.1 μM for GusA-HIS. The obtained data was evaluated and finally fitted to Michaelis-Menten-Kinetics with SigmaPlot™:
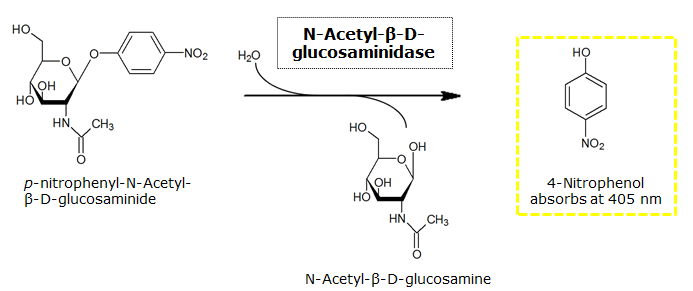
β-N-Acetylglucosaminidase (NagZ)
NagZ was proposed to be constitutively expressed in the mine colonies, processing its blue color-yielding substrate 5-Bromo-4-Chloro-3-indolyl-β-N-acetylglucosaminide (X-GluNAc), as shown in Figure 25. However, we have not managed to get color from the reaction with this enzyme-substrate pair. According to Orenga et al. (1), the substrate incorporating the indoxyl chromophore is less preferentially hydrolyzed by bacterial NagZ in comparison to yeast NagZ, and most preferentially hydrolyzes the alizarine-based substrate they applied. Using p-nitrophenyl-β-N-acetylglucosaminide (pNP-GluNAc), color assays in liquid culture, with the substrate end concentration of 0.01 mM, have shown development of yellow color, confirming that NagZ is expressed in our mine cells.
Crosstalk
To ensure orthogonality between the enzyme-substrate reactions used in Colisweeper, a crosstalk test has been done to ensure that all overexpressed enzymes specifically cleave their assigned substrate.
Color overlay
To play Colisweeper, colors for each colony identity have to be clearly distinguishable by eye. Because we are using high-pass filters to differentially process certain AHL levels, we had to make sure that a mix of cleaved product would show distinguishable colors.
References
(1) Orenga S, Roger-Dalbert C, James A, Perry J, US Patent 20090017481 (2009).
Absorption: Biosynth
 "
"