Team:ETH Zurich/Modeling/Reaction Diffusion OOHL
From 2013.igem.org
| Line 36: | Line 36: | ||
<br clear="all"/> | <br clear="all"/> | ||
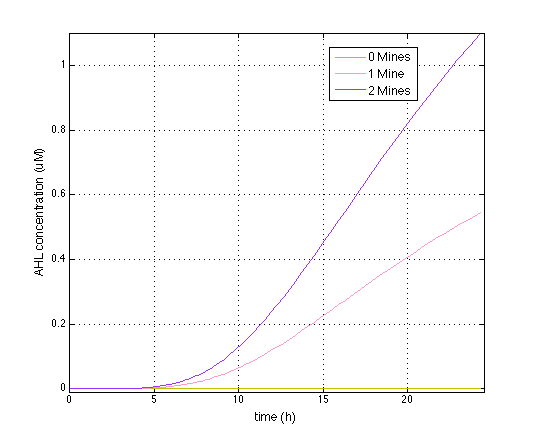
| - | [[File:Diff_ahl_gfp.png| | + | [[File:Diff_ahl_gfp.png|480px|left|thumb|<b>Figure 2:</b> averaged OHHL concentration of colonies with 0, 1 or 2 mines cells in the immediate vicinity, in μ M. Colony diameter: 5 mm.]] |
| - | [[File:AHL_ss.png| | + | [[File:AHL_ss.png|480px|left|thumb|<b>Figure 1:</b> 1D OHHL steady state concentration in radial direction, in mol/m<sup>3</sup>.]] |
| - | + | ||
| + | <br> | ||
<p align="justify">As can be seen, the model predicts that mine cells are able to generate a gradient by synthesizing OOHL at a sufficient rate to overcome the decay.</p> | <p align="justify">As can be seen, the model predicts that mine cells are able to generate a gradient by synthesizing OOHL at a sufficient rate to overcome the decay.</p> | ||
| + | <br clear="all"/> | ||
Revision as of 09:02, 1 October 2013
OHHL: Reaction-diffusion equations
Modelling the OHHL diffusion is important to get insights about time scale and distances, which plays an important role in determining the geometric setup of the playing field.
Video 1: 2D gradient formation over 24 hours, starting from steady state OHHL concentration in the mine cells. Display: 2D OHHL concentration in mol/m3. Geometric grid: 1 cm.
The change of OHHL concentration over time is influenced by two processes: (i) local chemical reactions and (ii) diffusion; which causes the molecule to spread over the agar plate. (Eq. 1).
For diffusion, we have a partial differential equation (Eq. 2) which describes density fluctuations over time and space (r=(x,y)). We do not model OHHL diffusion in and out cells explicitly; the underlying assumption is that this process is fast or that the molecule freely diffuses. From equation 2, DOHHL(OHHL(r,t),r) denotes the collective diffusion coefficient for OHHL at location r. However, we assume that the diffusion coefficient does not depend on the density, i.e., DOHHL is a constant. The value reported in the literature for the diffusion constant corresponds to measurements performed in water at 25oC. Since diffusion in our system happens in agar, we scaled the diffusion constant by a factor Cagar (Fatin-Rouge et al., 2004).
For the reaction component, the change of OHHL concentration over time is given by an ordinary differential equation (ODE), that comprises production and linear degradation or decay. However, the types of reactions that take place depend on the localization, i.e. extracellular or cytoplasmic; in the latter case, we have to further distinguish between the two type of cells encountered in our system, sender cells or so called mine cells and receiver cells. Mine cells synthesized the signalling molecule which depends on the product of luxI gene, while for the degradation we assumed a linear dependency of the molecule concentration. For the receiver cells only linear degradation take place, and we assume that is under the same rate as mine cells. However, for the extracellular decay we consider that OHHL degrades at a different rate, because the intracellular process is driven by enzymatic degradation, whereas the extracellular decay is a non active process. Furthermore, we include a dilution factor to take into account the cell growth (Eq. 3).
Finally, we need to specify the initial conditions (at time t = 0) and boundary conditions. At the starting point there is no OHHL in the agar plate, thus the initial concentration is zero ([OHHL(r,t=0)] = 0 M). For the boundary condition, we take into account that there is not flux out of the agar plate.
Results
In Figure 1 is displayed the time course of OHHL concentration; averaged for colonies surrounded by 0, 1 or 2 mine colonies. Simulations were initialized from the steady state concentrations.
As can be seen, the model predicts that mine cells are able to generate a gradient by synthesizing OOHL at a sufficient rate to overcome the decay.
 "
"