Team:NYMU-Taipei/Project/safe
From 2013.igem.org


Contents |
Eco-friendly E. coli
Introduction
Our Bee. coli can express antimicrobial peptides such as defensin and abaecin to fight against Nosema cerenae. However, it is also possible that our Bee. coli can contaminate the natural environment and cause death to other species. Therefore, a light-induced lysis system was created to ensure our Bee. coli only lives inside of the bee.
Background
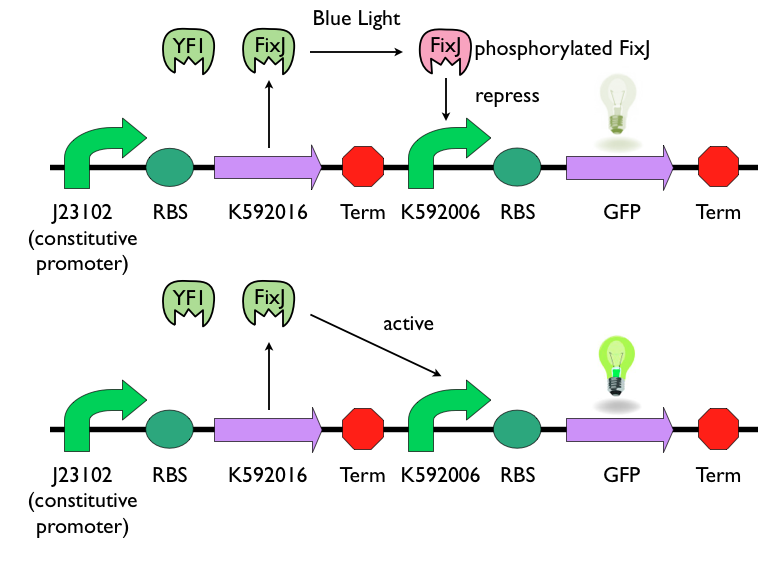
Blue Light Sensing Device
We chose to use K592016 and K592006 as our light sensing device. K592016 consists two parts: YF1 and FixJ. YF1 is a blue-light sensor protein. It works in conjunction with its response regulator, FixJ. K592006 is a light-sensing promoter, which can express downstream gene when unphosphorylated FixJ presents. When exposed to blue-light, YF1 will be activated and phosphorylate FixJ. Phosphorylated FixJ cannot activate the light-sensing promoter K592006, and the expression of downstream gene will be repressed. This picture can simply show the logic of this light-sensing device.
Self-destruct Device
The self-destruct device is composed of blue-light sensing promoter K592006, the blue-light sensing protein K592016, and a set of genes from toxin-antitoxin system. The toxin gene will be constitutively express. Antitoxin gene will be placed after K592006. When toxin and antitoxin gene are both expressed, antitoxin can repress the function of toxin and the bacteria can survive. However, when toxin gene is dominating, it will kill the bacteria.
Circuit design and Experimental Method
Circuit Design
K592016 is cloned after constitutive promoter J23102, so the proteins YF1 and FixJ were continuing produced. Toxin gene is also cloned after J23102 and will be constitutively produce. When not exposed to blue light, the inactive YF1 won't phosphorylate FixJ, and the unphosphorylated FixJ can induce the downstream gene of promoter K592006, in this case, which is the antitoxin gene. When not exposed to blue-light, antitoxin can be express and consequently repress the function of toxin. When exposed to blue-light, the expression of antitoxin will be repress, and constitutively produced toxin will kill Bee. coli.
Experimental Method
First, we substitute antitoxin with green fluorescent protein E0040 and replace toxin with red fluorescent protein E1010. By calculating the ratio of GFP and RFP, we can derive the expression ratio of toxin and antitoxin.
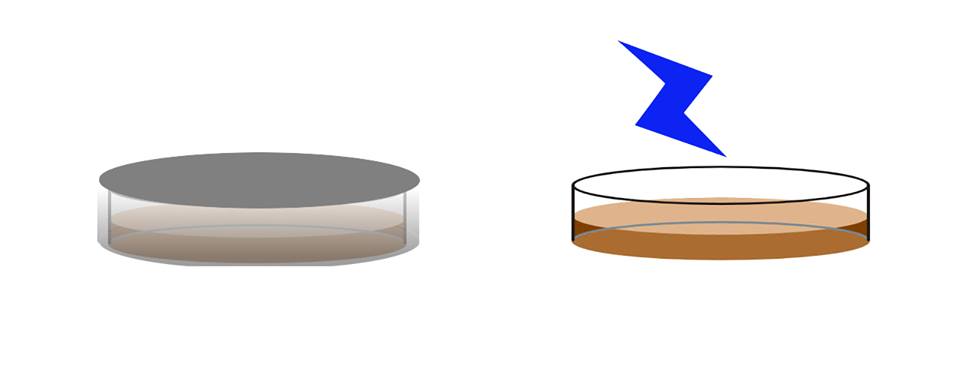
Next, by comparing the number of colonies of the plate that is exposed to light and the plate that is blocked from light after 16 hours of incubation, we can characterize the functions of our device.
This is the actual device we used to conduct this experiment.
 "
"