Team:Paris Saclay/Notebook/August/5
From 2013.igem.org
(Difference between revisions)
| Line 43: | Line 43: | ||
Damir, Nadia, XiaoJing | Damir, Nadia, XiaoJing | ||
| + | |||
| + | ===='''1 - Colony PCR of Bba_K1155004, Bba_K115605, Bba_K1155006 in DH5α'''==== | ||
| + | |||
| + | Damir, Nadia, XiaoJing | ||
| + | |||
| + | OU EST LA TRANFO PRECEDENTE ????????!!!!!!! | ||
| + | |||
| + | COLONIES PIQUEES DANS 10µL d'eau par Tube !!!!!!!!!!!!! | ||
| + | |||
| + | Used quantities : | ||
| + | * DNA : 2µL | ||
| + | * Mix : (it was divided in 25 tubes with 23µL of mix in each on) | ||
| + | ** Oligo 44 : 3.5µL | ||
| + | ** Oligo 45 : 3.5µL | ||
| + | ** Buffer Dream Taq : 70µL | ||
| + | ** dNTP : 28µL | ||
| + | ** Dream Taq : 5µL | ||
| + | ** H2O : 590µL | ||
| + | |||
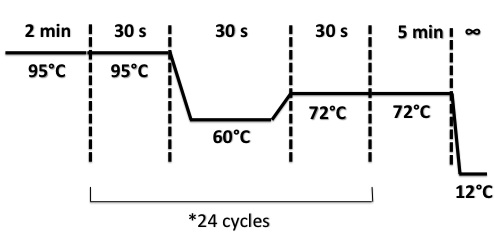
| + | PCR Program : | ||
| + | |||
| + | [[File:PsPCR0508.jpg|400px]] | ||
{{Team:Paris_Saclay/incl_fin}} | {{Team:Paris_Saclay/incl_fin}} | ||
Revision as of 23:20, 15 September 2013
Notebook : August 5
Lab work
A - Aerobic/Anaerobic regulation system
Objective : obtaining Bba_K1155003
1 - Digestion of Bba_K1155003 by EcoRI/PstI
Nadia, XiaoJing
Used quantities :
- Bba_K1155003 : 5µL
- EcoRI FD : 1µL
- PstI FD : 1µL
- Buffer FD : 3µL
- H2O : 20µL
We let the digestion at 37°C during 10 minutes ??????
2 - Electrophoresis of the digestion of Bba_K1155003
Damir
| File:Ps.jpg|350px]] |
|
Objective : obtaining Bba_K1155004, Bba_K1155005, Bba_K1155006
Damir, Nadia, XiaoJing
1 - Colony PCR of Bba_K1155004, Bba_K115605, Bba_K1155006 in DH5α
Damir, Nadia, XiaoJing
OU EST LA TRANFO PRECEDENTE ????????!!!!!!!
COLONIES PIQUEES DANS 10µL d'eau par Tube !!!!!!!!!!!!!
Used quantities :
- DNA : 2µL
- Mix : (it was divided in 25 tubes with 23µL of mix in each on)
- Oligo 44 : 3.5µL
- Oligo 45 : 3.5µL
- Buffer Dream Taq : 70µL
- dNTP : 28µL
- Dream Taq : 5µL
- H2O : 590µL
PCR Program :
 "
"