Team:Paris Saclay/Notebook/July/8
From 2013.igem.org
(Difference between revisions)
(→2 - Electrophoresis of the digestion of BBa_K592009, BBa_I732017 by NotI, XhoI, EcoRI, EcoRI/PstI) |
|||
| (12 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Team:Paris_Saclay/incl_debut_generique}} | {{Team:Paris_Saclay/incl_debut_generique}} | ||
| + | |||
='''Notebook : July 8'''= | ='''Notebook : July 8'''= | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
=='''Lab work'''== | =='''Lab work'''== | ||
| - | + | ==='''A - Aerobic/Anaerobic regulation system'''=== | |
| - | + | ||
| - | + | ===='''Objective : BBa_K1155003, BBa_K1155007'''==== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ===='''1 - Digestion of BBa_K592009, BBa_I732017 by NotI, XhoI, EcoRI, EcoRI/PstI'''==== | |
| - | + | Abou, Anaïs, Sheng, Zhou | |
| - | + | Used quantities : | |
| - | + | * NotI : | |
| - | + | ** BBa_K592009 : 2µL | |
| - | + | ** Buffer orange : 2µL | |
| - | * | + | ** NotI : 0.5µL |
| - | * | + | ** H2O : 15.5µL |
| - | * | + | |
| - | * | + | |
| - | * | + | |
| - | * | + | |
| - | * | + | |
| - | * | + | |
| - | * | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | * XhoI : | ||
| + | ** BBa_K592009 : 2µL | ||
| + | ** Buffer red : 2µL | ||
| + | ** XhoI : 0.5µL | ||
| + | ** H2O : 15.5µL | ||
| + | * EcoRI : | ||
| + | ** BBa_K592009 : 2µL | ||
| + | ** Buffer orange : 2µL | ||
| + | ** EcoRI : 0.5µL | ||
| + | ** H2O : 15.5µL | ||
| - | + | * EcoRI/PstI : | |
| - | + | ** BBa_I732017 : 2µL | |
| - | + | ** Buffer orange : 2µL | |
| + | ** EcoRI : 0.5µL | ||
| + | ** PstI : 0.5µL | ||
| + | ** H2O : 15µL | ||
| - | + | We let our digestion 1h30 at 37°C. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ===='''2 - Electrophoresis of the digestion of BBa_K592009, BBa_I732017 by NotI, XhoI, EcoRI, EcoRI/PstI'''==== | |
| - | + | ||
| - | + | ||
| - | + | Abdou, Anaïs, Sheng, Zhou | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | {| | |
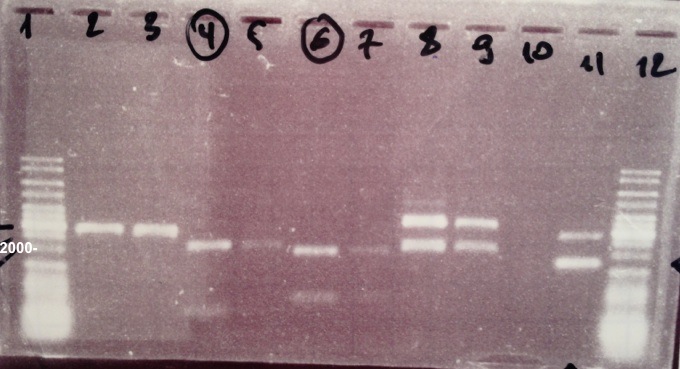
| - | * | + | | style="width:350px;border:1px solid black;" |[[File:Psgel0807.jpg|500px]] |
| + | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| + | * Well 1 : 6µL DNA Ladder | ||
| + | * Well 2 : 5µL BBa_K592009 clone 1 digested by EcoRI+1µL of 6X loading dye | ||
| + | * Well 3 : 5µL BBa_K592009 clone 2 digested by EcoRI+1µL of 6X loading dye | ||
| + | * Well 4 : 5µL BBa_K592009 clone 1 digested by NotI+1µL of 6X loading dye | ||
| + | * Well 5 : 5µL BBa_K592009 clone 2 digested by NotI+1µL of 6X loading dye | ||
| + | * Well 6 : 5µL BBa_K592009 clone 1 digested by XhoI+1µL of 6X loading dye | ||
| + | * Well 7 : 5µL BBa_K592009 clone 2 digested by XhoI+1µL of 6X loading dye | ||
| + | * Well 8 : 5µL BBa_I732017 clone 1 digested by EcoRI/PstI+1µL of 6X loading dye | ||
| + | * Well 9 : 5µL BBa_I732017 clone 2 digested by EcoRI/PstI+1µL of 6X loading dye | ||
| + | * Well 10 : - | ||
| + | * Well 11 : - | ||
| + | * Well 12 : 6µL DNA Ladder | ||
| + | * Gel : 1% | ||
| + | |} | ||
| - | + | Expected sizes : | |
| + | * BBa_K592009 digested by NotI : 2046bp + 693bp | ||
| + | * BBa_K592009 digested by XhoI : 1842bp + 892bp | ||
| + | * BBa_I732017 : 3093bp | ||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We obtain fragments at the right size. Our extraction was good. | ||
| + | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| border="1" align="center" | {| border="1" align="center" | ||
|[[Team:Paris Saclay/Notebook/July/5|<big>Previous week</big>]] | |[[Team:Paris Saclay/Notebook/July/5|<big>Previous week</big>]] | ||
| Line 197: | Line 82: | ||
|[[Team:Paris Saclay/Notebook/July/9|<big>Next day</big>]] | |[[Team:Paris Saclay/Notebook/July/9|<big>Next day</big>]] | ||
|} | |} | ||
| - | |||
| - | |||
{{Team:Paris_Saclay/incl_fin}} | {{Team:Paris_Saclay/incl_fin}} | ||
Latest revision as of 23:55, 4 October 2013
Contents |
Notebook : July 8
Lab work
A - Aerobic/Anaerobic regulation system
Objective : BBa_K1155003, BBa_K1155007
1 - Digestion of BBa_K592009, BBa_I732017 by NotI, XhoI, EcoRI, EcoRI/PstI
Abou, Anaïs, Sheng, Zhou
Used quantities :
- NotI :
- BBa_K592009 : 2µL
- Buffer orange : 2µL
- NotI : 0.5µL
- H2O : 15.5µL
- XhoI :
- BBa_K592009 : 2µL
- Buffer red : 2µL
- XhoI : 0.5µL
- H2O : 15.5µL
- EcoRI :
- BBa_K592009 : 2µL
- Buffer orange : 2µL
- EcoRI : 0.5µL
- H2O : 15.5µL
- EcoRI/PstI :
- BBa_I732017 : 2µL
- Buffer orange : 2µL
- EcoRI : 0.5µL
- PstI : 0.5µL
- H2O : 15µL
We let our digestion 1h30 at 37°C.
2 - Electrophoresis of the digestion of BBa_K592009, BBa_I732017 by NotI, XhoI, EcoRI, EcoRI/PstI
Abdou, Anaïs, Sheng, Zhou
Expected sizes :
- BBa_K592009 digested by NotI : 2046bp + 693bp
- BBa_K592009 digested by XhoI : 1842bp + 892bp
- BBa_I732017 : 3093bp
|
We obtain fragments at the right size. Our extraction was good. |
| Previous week | Back to calendar | Next day |
 "
"