Team:TU-Munich/Project/Bioaccumulation
From 2013.igem.org
m (→Protein Phosphotase 1 - A molecular mop for Microcystin) |
m (→References:) |
||
| Line 126: | Line 126: | ||
#[[http://www.ncbi.nlm.nih.gov/pubmed/16307475 Vopel et al., 2005]] Vopel S, Mühlbach H, Skerra A. (2005) Rational engineering of a fluorescein-binding anticalin for improved ligand affinity. ''Biol. Chem.'', 386(11):1097-104. | #[[http://www.ncbi.nlm.nih.gov/pubmed/16307475 Vopel et al., 2005]] Vopel S, Mühlbach H, Skerra A. (2005) Rational engineering of a fluorescein-binding anticalin for improved ligand affinity. ''Biol. Chem.'', 386(11):1097-104. | ||
#[[http://www.ncbi.nlm.nih.gov/pubmed/9255793 Nord et al., 1997]] Nord K, Gunneriusson E, Ringdahl J, Ståhl S, Uhlén M, Nygren PA. (1997) Binding proteins selected from combinatorial libraries of an α-helical bacterial receptor domain. ''Nature Biotech''. 15(8):772-7. | #[[http://www.ncbi.nlm.nih.gov/pubmed/9255793 Nord et al., 1997]] Nord K, Gunneriusson E, Ringdahl J, Ståhl S, Uhlén M, Nygren PA. (1997) Binding proteins selected from combinatorial libraries of an α-helical bacterial receptor domain. ''Nature Biotech''. 15(8):772-7. | ||
| + | #[[http://toxics.usgs.gov/definitions/bioaccumulation.html Definition of Bioaccumulation,U.S. Department of the Interior | U.S. Geological Survey,2013]] | ||
| + | #[[http://www.ncbi.nlm.nih.gov/pubmed/15676296 Schlehuber and Skerra,2005]] Schlehuber S, Skerra A.(2005)Lipocalins in drug discovery: from natural ligand-binding proteins to "anticalins".10(1):23-33 | ||
</div> | </div> | ||
Revision as of 12:29, 18 September 2013
BioAccumulation
BioAccumulation
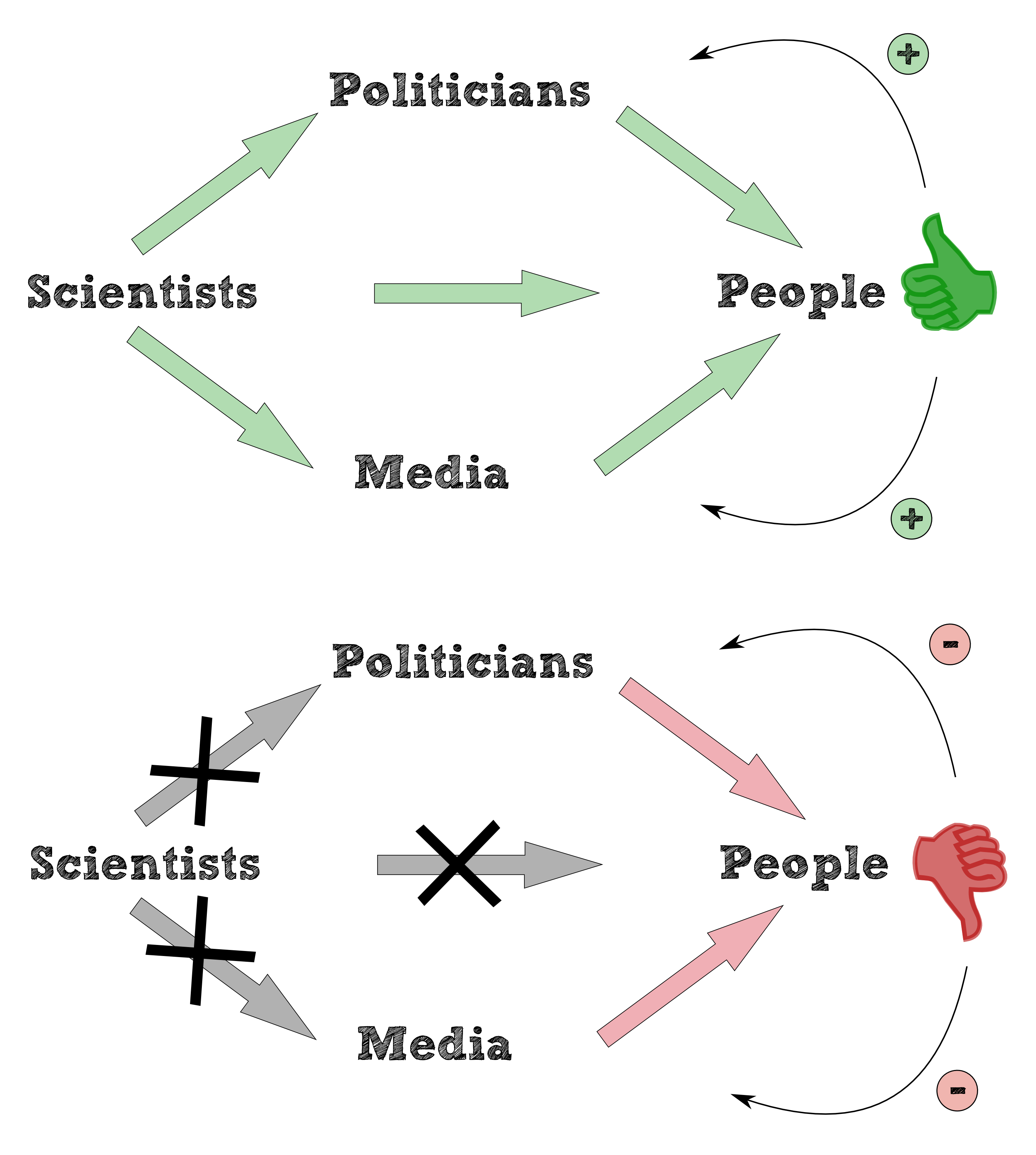
In general bioaccumulation is a versatile and ambiguous concept to be defined. “General term describing a process by which chemicals are taken up by an organism either directly from exposure to a contaminated medium or by consumption of food containing the chemical.” is the definition of bioaccumulation of the [http://toxics.usgs.gov/definitions/bioaccumulation.html U.S. Environmental Protection Agency, 2010]. The majority of definitions is aimed at the vicious circle of the predator-prey relationship caused by the bioaccumulation shows the build up of persistent chemicals such as DDT, PBC or dioxins in aquatic and terrestrial organisms. Health problems in humans, the survival of some affected populations and overall biodiversity in aquatic and terrestrial ecosystems are at risk and a result of the consume and the accumulation of persistent chemicals along the food chain (see figure 1 ). The Minamata catastrophe 1950 substantiates the result of mecury poisoning: the Chisso Corporation in Minamata released untreated effluent containing methylmercurychloride into Minamata Bay.
In Respect to Bioaccumulation we propose the employment of transgenic moos (Physcomitrella patens ) to produce effectors for enzymatic specific binding of pollutants additional to the biodegradation method for the development of a functional wastewater filter system which convert noxious critical substances in wastewater into non-hazardous compounds .Bioaccumulation is a possibility to remove xenobiotics from the environment by binding them to a protein that has been designed for this purpose (see figure 2). There are a broad range of natural as well as engineered binding proteins available. Natural binding proteins act as a model and initiator in design of new artificial binding proteins regarding research in different fields of biotechnology. Lipocalins, natural binding proteins, as base and scaffold for the design of anticalins confirms the popularity of bioaccumulated proteins in red biotechnologyhttp://www.ncbi.nlm.nih.gov/pubmed/15676296 Schlehuber and Skerra, 2005 . The role of Fibrillin in the abscisic acid-mediated photoprotection shows an example of functioned bioaccumulation in a plant http://www.pnas.org/content/103/15/6061 Yang and Grill et al.,2007. The most commonly known binding proteins are antibodies which defend mammals against pathogens and toxins. Beside these natural binding proteins and anticalins there are more and more designed binding proteins such as Affibodies derived from the z-domain of the antibody-binding protein A (Ref) and DARPins that are based on an ankyrin scaffold .Critical substances such as Bisphenol A known as an endocrine disrupter which can mimic endrogen and the anti-inflammatory drug used as analgesic Diclofenac could accumulate by our physco filter. The last effects in contrast to the negative health effects developed from Bisphenol A ecological effects like on freshwater fish species. The localization of the effectors is exclusive membrane associated in contrast to the cytoplasmatic or secretory localization by biodegraded effectors.
The Fluorescein binding Anticalin FluA
Mostly in biochemical research applications a synthetic organic dark red powder is used to label and track cells as well as target to specific proteins or structures within cells. Stunningly that this red coloured powder was the reason for the green dye of the http://www.greenchicagoriver.com/ Chicago River on St. Patrick’s Day on Ireland in 1962. Meant is the well-known fluorophore fluorescein which was used from the [team Freiburg 2008] to demonstrate the high expression level of the fusion protein and the localization at the cell membrane obvious by the strong fluorescent signal at the cell surface for their Modular Synthetic Receptor System. To develop its fluorescence character an artificial designed binding protein was necessary. The choice fell on the lipocalin fluA which variants of the fluorescein binding confirms table 1. To prove the extracellular membranbinding localization of our constructs in context of bioremediation the interaction between fluA and flourescein was chosen . In contrast to iGEM Freiburg we used another biobrick for flu A based on the necessarily of higher dissociation constant for our Physco filter system (see table 1). So we created a transgenic moss plants ([PF-15]) with the fluorescein binding anticalin flu A on the extracellular part of the receptor . Producing engineered proteins for medical purpose in the red biotechnology is one of the main research fields in which anticalins play a major role. Lipocalins such as bilin-binding-protein BBP (from Pieris brassicae) can be used for generating molecular pockets with a diversity of shapes and for creating a stable receptor protein for a ligand of choice, so that development of binding proteins against nearly chemical structures with comparable size is possible. Figure 3 shows the principle of reshape the binding pocket by amino acid substitutions in context of the conversion from BBP to flu A. The binding site of the αβ-scaffold of BBP is formed by four loops on the top of an eight-standed β-barrel. To recognize fluorescein instead of bilin 16 residues in the binding center were encountered a random mutagenesis. Ligand binding studies and mutagenesis experiments shown the specificity of the molecular recognition of fluorescein through hydrophobic packing, charged sidechain environment and hydrogen bonds with its hydroxyl- groups, the responsibility for tight complex formation by charged residues at the pocket center and the variability of the randomizes amino acid positions. The specificity is encoded in the first shell residues of the binding pocket shows the following publication in which the swap of binding specificity happened via binding pocket grafting http://www.ncbi.nlm.nih.gov/pubmed/23792166 Scheib et al.,2013 . In addition to the changed loop region which leads to a deeper cavity of fluorescein than bilin the conformation of the base of the binding pocket shows a rearrangement. The electron transfer can be explained with the interaction between Trp 129 with the xanthenolone part http://www.pnas.org/content/96/5/1898.full.pdf Beste and Skerra et al.,1999 http://onlinelibrary.wiley.com/doi/10.1002/prot.10497/abstract Korndörfer,Skerra,Beste,2003.
Table 1:
Variants of the fluorescein binding Anticalin FluA | |||
| Proteinvariant | KD of FluA to fluorescein | Literature reference | BioBrick |
| FluA | 152 nM | http://www.ncbi.nlm.nih.gov/pubmed/10051566 Beste et al., 1999 | <partinfo>BBa_K157004</partinfo> |
| FluA (R95K) | 64 nM | http://www.ncbi.nlm.nih.gov/pubmed/16307475 Vopel et al., 2005 | not availible as BioBrick |
| FluA (R95K, A45I, S114T) | 2 nM | http://www.ncbi.nlm.nih.gov/pubmed/16307475 Vopel et al., 2005 | <partinfo>BBa_K1159002</partinfo> |
Glutathione S-transferase
Glutathion S-transferases (GSTs), an eukaryotic and prokaryotic phase II metabolic isozymes-family, catalyze the conjugation of reduced form of gluthatione (GSH, nucleophil) and xenobiotic (electrophil) to gluthatione-S-Conjugate via nucleophilic attack (see figure 4). The consequence is an increased solubility of the conjugates which leads to the removal of xenobiotics in form of conjugates via vacuole enclosure or excretion. The action of the specific transporters and the steady supply of GSH in the equilibrium reaction (see figure 5) are the limited factors of the detoxification reaction. Along with the detoxification and cell signaling function GST’s act as transport proteins, which gave GST the previous name ligandin. Table 2 shows the 3 different superfamilies with their characteristics.Generally the three superfamilies differ mostly in structure and sequence why just cytosolic and the mitochondrial superfamily have a thiorexin like domain in which the glutathione binding site (G-site) is located http://www.ncbi.nlm.nih.gov/pubmed/21428697 Oakley A.,2011. The helix alpha 2 is the most variable secondary structure. Y-GST is the subgroup which activates glutathione via using tyrosine residues. S/C-GST uses serine/cysteine residues. GST binds the substrate at the hydrophobic H-site of the enzyme and GSH at the hydrophilic G-site which together form the active site of the enzyme(see figure 6). In research techniques GST will be used as so called GST-tags for separation, elucidation of direct protein-protein interaction and purification of the GST-fusion protein mostly by pull-down assay. So targeting GST with molecule therapeutics represents GTS as an attractive target for drug discovery http://www.ncbi.nlm.nih.gov/pubmed/16550164 McIlwain CC, Townsend DM, Tew KD,2006. A mammalian variant of GST, GSTP, plays a major role in cancer- development and potential drug/chemotherapeutic resistance in a majority of tumor cell lines: The inhibition of the pro-apoptotic pathway (JNK pathway) and the overexpression of GSTP in tumor cells lead to escape of apoptosis of the tumor cells mediated by non GSTP- substance-drugs http://www.ncbi.nlm.nih.gov/pubmed/8770536 Hayes and Pulford,2005 http://www.ncbi.nlm.nih.gov/pubmed/20981235 Josephy,2010 http://www.ncbi.nlm.nih.gov/pubmed/10971201 Hayes and Strange,2000 http://www.ncbi.nlm.nih.gov/pubmed/12563680 Fraser et al.,2003.In context to prove the bioaccumulation we use the cytoplasmatic GST 1-1 also known as DDT Dehyrochlorinase. Dichlordiphenyltrichlorethane (DDT, from Anopheles dirus mosquito) is an endocrine disrupter and persistent organic pollutant http://www.sciencedirect.com/science/article/pii/0965174895000909 La-Aied Prapanthadara,1996. To avoid the time- and labor-intensive method HPLC following derivatization with 2-nitrobenzoic acid we used the common sensitive technique with monochlorobimane to measure GSH as a proof of principle. The adding of monochlorobimane to the culture medium leads to the conjugation of GSH to monochlorobimane catalyzed by DDT(see figure 7). The GSH-monochlorobimane conjugate can be measured fluorometrically http://www.ncbi.nlm.nih.gov/pubmed/11038270 Kamencic et al.,2000 . Along with the creation of transgenic Physcomitrella patens plants (PF-13 ) we used the biobrick from the Caltech iGEM team 2011 . This team engineered bacteria which can degrade endocrine-disrupting chemicals such as DDT, synthetic estrogen in bodies of water to less toxic forms.
Table 1:
superfamilies of GST with their characteristics | |||
| Superfamily of GTS | Classes based upon their structure | Sequence homology [%] | |
| Cytosolic proteins | alpha, beta, delta, epsilon, zeta, theta, mu, nu, pi, sigma, tau, phi, and omega | >40 | |
| Mitochondrial proteins | kappa | <25 | |
| microsomal (MAPEG= membrane-associated proteins in eicosanoid and glutathione metabolism) proteins | subgroups I-IV | <25 | |
Protein Phosphotase 1 - A molecular mop for Microcystin
The initiator of fixing phosphate residues to proteins is an enzyme family called kinases. The adversaries of the phosphorylation process are the protein phosphatases. In general you classify them in three families: phosphoserine and phosphothreonine residues were dephosporylated by PPM and PPP families whereas PTP dehosphorylate phosphotyrosine amino acids. The role of regulation of cellular processes represent PP1 which is an ubiquitous eukaryotic enzyme belonging to the protein serine/threonine phosphatase class. The enormous variety and multifunctionality of PP1 concerning to cellular functions through the interaction of its catalytic subunit (PP1c) is written down in the publication "Functional diversity of protein phosphatase-1, a cellular economizer and reset button" [http://www.ncbi.nlm.nih.gov/pubmed/14715909 Ceulemans and Bollen,2004]. Next to wide diversity of biological function such as the regulation of blood-glucose levels and glycogen metabolism PP1 plays a major relevance in disease research which aims in the red biotechnology to produce new drug designs. The role of PP1 concerning to the HIV-1-transcription http://www.ncbi.nlm.nih.gov/pubmed/17266553 Nekhai et al.,2007 and Alzheimer disease brains http://www.ncbi.nlm.nih.gov/pubmed/8395566 Gong et al.,1993 illustrate this. Basically changes in the levels, phosphorylation status and conformation of the PP1c which consists of diverse regulatory subunits and domains hydrophobic grooves on the surface mostly through a short conserved binding motif RVxF leads to targeting of substrates and inhibitors. Together with the conserved structure figure 8 confirms the metalloenzyme characteristic of PP1: next to the potential substrate/inhibitor binding grooves with the C-Terminus at the end of the grooves a central β-α-β-α-β scaffold at the active site positions the two metal ions (Mn, Fe) which are coordinated by one asparagines, two aspartic acids and three histidines http://www.nature.com/nature/journal/v376/n6543/abs/376745a0.html Goldberg,2002.An useable application of PP1 with regard to bioremediation implicates the iGEM project 2013 of team Dundee. PP1 as a natural binding protein accumulates the toxin microcystin(toxin released by Microcystis aeruginosato) combat the releases harm to mammals. Next to this microcystin plays also a role in the modulation of proliferation, apoptosis and proliferation of spermatogenic cells in vivo http://www.ncbi.nlm.nih.gov/pubmed/24025782 Zhou et al.,2013. The interaction of microcystin in form of the heptapeptide structure with three distinct regions of the PP1c shows figure 9. After converting the human PP1, given by iGEM Dundee, in RFC 25 and constructing some expression plasmids we produced transgenic phytomitrella patens (PF-14) with PP1 and a biobrick of PP1 receptor.
References:
- http://www.ncbi.nlm.nih.gov/pubmed/10051566 Beste et al., 1999 Beste G, Schmidt FS, Stibora T, Skerra A. (1999) Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold. PNAS, 96(5):1898-903.
- http://www.ncbi.nlm.nih.gov/pubmed/16307475 Vopel et al., 2005 Vopel S, Mühlbach H, Skerra A. (2005) Rational engineering of a fluorescein-binding anticalin for improved ligand affinity. Biol. Chem., 386(11):1097-104.
- http://www.ncbi.nlm.nih.gov/pubmed/9255793 Nord et al., 1997 Nord K, Gunneriusson E, Ringdahl J, Ståhl S, Uhlén M, Nygren PA. (1997) Binding proteins selected from combinatorial libraries of an α-helical bacterial receptor domain. Nature Biotech. 15(8):772-7.
- U.S. Geological Survey,2013
- http://www.ncbi.nlm.nih.gov/pubmed/15676296 Schlehuber and Skerra,2005 Schlehuber S, Skerra A.(2005)Lipocalins in drug discovery: from natural ligand-binding proteins to "anticalins".10(1):23-33
 "
"



AutoAnnotator:
Follow us:
Address:
iGEM Team TU-Munich
Emil-Erlenmeyer-Forum 5
85354 Freising, Germany
Email: igem@wzw.tum.de
Phone: +49 8161 71-4351