Team:TU-Munich/Project/Bioaccumulation
From 2013.igem.org
BioAccumulation
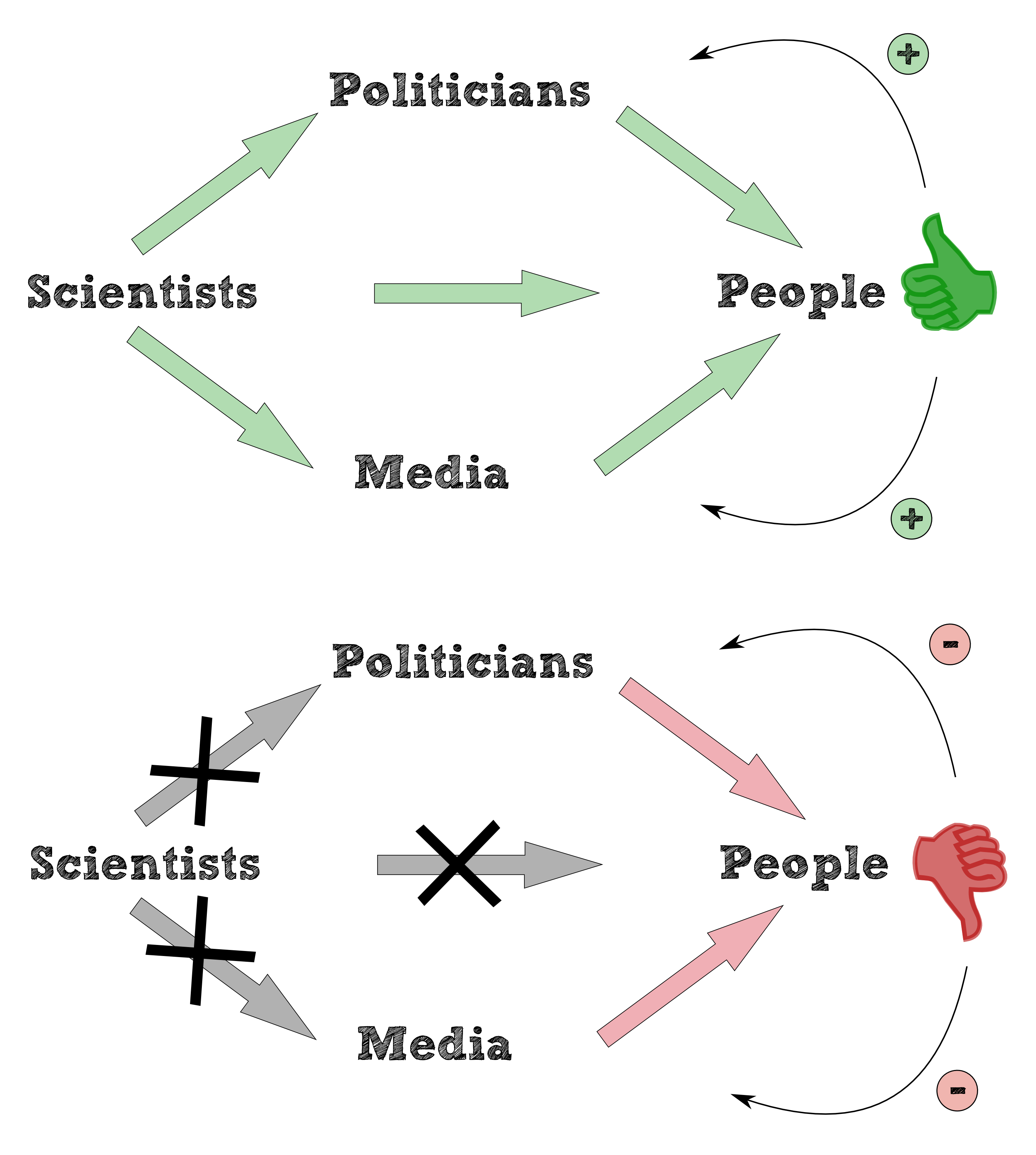
Bioaccumulation is a versatile and ambiguous concept to be defined. “General term describing a process by which chemicals are taken up by an organism either directly from exposure to a contaminated medium or by consumption of food containing the chemical.” is the definition of bioaccumulation of the [http://toxics.usgs.gov/definitions/bioaccumulation.html U.S. Environmental Protection Agency, 2010]. The majority of definitions is aimed at the vicious circle of the predator-prey relationship caused by the bioaccumulation which shows the build up of persistent chemicals such as DDT, PBC or dioxins in aquatic and terrestrial organisms. Health problems in humans, the survival of some affected species and overall biodiversity in aquatic and terrestrial ecosystems are at risk and a result of the consume and the accumulation of persistent chemicals along the food chain (see Figure 1). As most of these persistent chemicals are not biodregadable, an other approach is necessary.
Using our transgenic moos (Physcomitrella patens ) as chassis, we can produce effectors for specific binding of pollutants. For the sustained removal of the target pollutant the localization of the effectors must be membrane associated in contrast to the cytoplasmatic or secretory localization of biodegrading effectors. The membrane association ensures the fixation of the pollutant to the membrane after successful binding. Internalisation of the effectors either results in metabolisation or increased targeted bioaccumulation of the pollutant in the transgenic plant cell. In case of bioaccumulation of the pollutant, the regular, safe disposal of parts of the plants contained in the filter would be necessary.
There is a broad range of natural as well as engineered binding proteins available. Natural binding proteins act as a model and initiator in design of new artificial binding proteins regarding research in different fields of biotechnology. Lipocalins, natural binding proteins, as base and scaffold for the design of anticalins confirms the popularity of bioaccumulated proteins in red biotechnology http://www.ncbi.nlm.nih.gov/pubmed/15676296 Schlehuber et al., 2005. The role of Fibrillin in the abscisic acid-mediated photoprotection shows an example of functioned bioaccumulation in a plant http://www.pnas.org/content/103/15/6061 Yang et al.,2007. The most commonly known binding proteins are antibodies which defend mammals against pathogens and toxins. Beside these natural binding proteins and anticalins there are more and more designed binding proteins such as Affibodies derived from the z-domain of the antibody-binding protein A (Ref) and DARPins that are based on an ankyrin scaffold.One research topic of the [http://www.ws.chemie.tu-muenchen.de chair of analytical chemistry of the TU Munich] is the depletion of algal toxin contaminated water by using selective biofilters based on plantibodies. Plantibodies are plant- produced and derived antibodies which can construct antibody-mediated pathogen resistance as well as change the plant phenotype by immunomodulation http://www.ncbi.nlm.nih.gov/pubmed/11950570 Stoger et al.,2002.
For our project we exemplarily chose three different binding proteins which each exploit different mechanisms to bind Proteins.
- fluA as an example for an anticalin
- Gluthathione-S-Transferase as an example where covalent bonds between the target pollutant and another molecule (gluthatione) are created
- Protein Phosphatase 1 which exploits the inhibitory binding of the pollutant to the enzyme
Bioaccumulation via binding proteins
Problem Example: Diclofenac
Are pharmaceuticals a blessing or a curse? Respective to the ecological consequences of diclofenac this task must be valued negative. Diclofenac which is a proven, nonsteroidal anti-imflammatory drug (NSAID) is used mostly in the human medicine to treat a variety of acute and chronic pain and inflammatory conditions via inhibition of prostaglandin synthesis by inhibiting cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2)http://www.ncbi.nlm.nih.gov/pubmed/20470236 Gan,2010. Its necessity and importance in medicine research confirms e.g. the development of diclofenac patches for topical treatment of acute impact injuries http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1724805/ Predel et al.,2004 and the main studies about safety and efficiency of the analgesic, anti-inflammatory, and antipyretic properties of diclofenac http://www.ncbi.nlm.nih.gov/pubmed/11276273 Morgan et al.,2001http://www.ncbi.nlm.nih.gov/pubmed/15480981 Chan et al.,2004 http://www.google.de/url?sa=t&rct=j&q=&esrc=s&source=web&cd=9&ved=0CHsQFjAI&url=http://www.ncbi.nlm.nih.gov/pubmed/22168216&ei=DLI5UqrPNJHSsgaL2YHAAQ&usg=AFQjCNFs7U5eWUrOKgcIuLly-afQqXpwTQ&sig2=ebhoYAq_NdG9v2nuxdB8BQ&bvm=bv.52288139,d.Yms Pavelka,2012. The possible entered vicious circle (see figure 1) shows the poisoning tragedy in 2005 which leads to mass mortality and decline of vultures species especially Gyps bengalensis, Gyps indicus and Gyps tenuirostris across the Indian sub-continent. Based on the consume of diclofenac by animal cadaver which are the main food source of vultures is the result of the eradication, breeding programs and reduction of the species diversity.
Mostly in biochemical research applications a synthetic organic dark red powder is used to label and track cells as well as target to specific proteins or structures within cells. Stunningly that this red colored powder was the reason for the green dye of the http://www.greenchicagoriver.com/ Chicago River on St. Patrick’s Day on Ireland in 1962. Meant is the well-known fluorophore fluorescein which was used by the [team Freiburg 2008] to demonstrate the high expression level of the fusion protein and the localization at the cell membrane obvious by the strong fluorescent signal at the cell surface for their Modular Synthetic Receptor System. To develop its fluorescence character the artificial designed binding protein flu A, which variants of the fluorescein binding confirms table 1, was chosen. To prove the extracellular membranbinding localization of our constructs in context of bioremediation the interaction between fluA and flourescein was chosen . In contrast to iGEM Freiburg we used another biobrick for flu A based on the necessarily of higher dissociation constant for our Physco filter system (see table 1). So we created a transgenic moss plants ([PF-15]) with the fluorescein binding anticalin flu A on the extracellular part of the receptor . Producing engineered proteins for medical purpose in the red biotechnology is one of the main research fields in which anticalins play a major role. Lipocalins such as bilin-binding-protein BBP (from Pieris brassicae) can be used for generating molecular pockets with a diversity of shapes and for creating a stable receptor protein for a ligand of choice, so that development of binding proteins against nearly chemical structures with comparable size is possible. Figure 3 shows the principle of reshape the binding pocket by amino acid substitutions in context of the conversion from BBP to flu A. The binding site of the αβ-scaffold of BBP is formed by four loops on the top of an eight-standed β-barrel. To recognize fluorescein instead of bilin 16 residues in the binding center were encountered a random mutagenesis. Ligand binding studies and mutagenesis experiments shown the specificity of the molecular recognition of fluorescein through hydrophobic packing, charged sidechain environment and hydrogen bonds with its hydroxyl- groups, the responsibility for tight complex formation by charged residues at the pocket center and the variability of the randomizes amino acid positions. The specificity is encoded in the first shell residues of the binding pocket shows the following publication in which the swap of binding specificity happened via binding pocket grafting http://www.ncbi.nlm.nih.gov/pubmed/23792166 Scheib et al.,2013 . In addition to the changed loop region which leads to a deeper cavity of fluorescein than bilin the conformation of the base of the binding pocket shows a rearrangement. The electron transfer can be explained with the interaction between Trp 129 with the xanthenolone part http://www.pnas.org/content/96/5/1898.full.pdf Beste et al.,1999 http://onlinelibrary.wiley.com/doi/10.1002/prot.10497/abstract Korndörfer et al.,2003.
Table 1:
Variants of the fluorescein binding Anticalin FluA | |||
| Proteinvariant | KD of FluA to fluorescein | Literature reference | BioBrick |
| FluA | 152 nM | http://www.ncbi.nlm.nih.gov/pubmed/10051566 Beste et al., 1999 | <partinfo>BBa_K157004</partinfo> |
| FluA (R95K) | 64 nM | http://www.ncbi.nlm.nih.gov/pubmed/16307475 Vopel et al., 2005 | not availible as BioBrick |
| FluA (R95K, A45I, S114T) | 2 nM | http://www.ncbi.nlm.nih.gov/pubmed/16307475 Vopel et al., 2005 | <partinfo>BBa_K1159002</partinfo> |
Glutathione S-transferase
Problem: Dichlorodiphenyltrichloroethane
Since the entry into force of the Stockholm Convention in 2004 the insecticide Dichlordiphenyltrichlorethan (DDT) is just allowed for the abatement of disease-carrying insects such as the malaria carrying Anopheles dirus mosquitohttp://www.sciencedirect.com/science/article/pii/0965174895000909 La-Aied Prapanthadara et al.,1996. This measure was caused by the devastating consequences for the ecosystem. DDT’s property as an endocrine disrupter of the periphery nervous system leads to the inhibition of the Na+ canal lock in the repolarisation phase. The dilution of the eggshell of raptor is one result of the DTT effect in wildlife. The poverty of organic farmer is another negative effect of DDT in addition to the health damage of humans http://www.spiegel.de/wissenschaft/natur/insektengift-ddt-wie-die-malaria-wunderwaffe-bauern-in-die-armut-treibt-a-768654.html Koch L.,2011 http://www.ncbi.nlm.nih.gov/pubmed/16818570 Eskenazi et al.,2006 . Because of its stability and capacity to accumulate in human adipose tissue the risk of cancer increases http://www.ncbi.nlm.nih.gov/pubmed/11836138 Turusov et al.,2002 .
Caltech 2011 Solution: Covalent binding to Gluthathion via Gluthathione-S-Transferase
In context to prove the bioaccumulation we use the cytoplasmatic GST 1-1 also known as DDT Dehyrochlorinase. Along with the creation of transgenic Physcomitrella patens plants (PF-13 ) we used the biobrick from the Caltech iGEM team 2011. This team engineered bacteria which can degrade endocrine-disrupting chemicals such as DDT, synthetic estrogen in bodies of water to less toxic forms.Glutathion S-transferases (GSTs), an eukaryotic and prokaryotic phase II metabolic isozymes-family, catalyze the conjugation of reduced form of gluthatione (GSH, nucleophil) and xenobiotics (electrophil) to gluthatione-S-Conjugate via nucleophilic attack (see figure 4). The consequence is an increased solubility of the conjugates which leads to the removal of xenobiotics in form of conjugates via vacuole enclosure. The action of the specific transporters and the steady supply of GSH in the equilibrium reaction (see figure 5) are the limited factors of the detoxification reaction. Along with the detoxification and cell signaling function GST’s act as transport proteins, which gave GST the previous name ligandin. Table 2 shows the 3 different superfamilies with their characteristics.Generally the three superfamilies differ mostly in structure and sequence as only the cytosolic and the mitochondrial superfamily have a thiorexin like domain in which the glutathione binding site (G-site) is located http://www.ncbi.nlm.nih.gov/pubmed/21428697 Oakley A.,2011. The helix alpha 2 is the most variable secondary structure. Y-GST is the subgroup which activates glutathione via using tyrosine residues. S/C-GST uses serine/cysteine residues. GST binds the substrate at the hydrophobic H-site of the enzyme and GSH at the hydrophilic G-site which together form the active site of the enzyme(see figure 6). In research techniques GST will be used as so called GST-tags for separation, elucidation of direct protein-protein interaction and purification of the GST-fusion protein mostly by pull-down assay. So targeting GST with molecule therapeutics represents GTS as an attractive target for drug discovery http://www.ncbi.nlm.nih.gov/pubmed/16550164 McIlwain et al.,2006. A mammalian variant of GST, GSTP, plays a major role in cancer- development and potential drug/chemotherapeutic resistance in a majority of tumor cell lines: The inhibition of the pro-apoptotic pathway (JNK pathway) and the overexpression of GSTP in tumor cells lead to escape of apoptosis of the tumor cells mediated by non GSTP- substance-drugs http://www.ncbi.nlm.nih.gov/pubmed/8770536 Hayes et al.,2005 http://www.ncbi.nlm.nih.gov/pubmed/20981235 Josephy,2010 http://www.ncbi.nlm.nih.gov/pubmed/10971201 Hayes et al.,2000 http://www.ncbi.nlm.nih.gov/pubmed/12563680 Fraser et al.,2003. To avoid the time- and labor-intensive method HPLC following derivatization with 2-nitrobenzoic acid we used the common sensitive technique with monochlorobimane to measure GSH as a proof of principle. The adding of monochlorobimane to the culture medium leads to the conjugation of GSH to monochlorobimane catalyzed by DDT(see figure 7). The GSH-monochlorobimane conjugate can be measured fluorometrically http://www.ncbi.nlm.nih.gov/pubmed/11038270 Kamencic et al.,2000 .
Table 1:
superfamilies of GST with their characteristics | |||
| Superfamily of GTS | Classes based upon their structure | Sequence homology [%] | |
| Cytosolic proteins | alpha, beta, delta, epsilon, zeta, theta, mu, nu, pi, sigma, tau, phi, and omega | >40 | |
| Mitochondrial proteins | kappa | <25 | |
| microsomal (MAPEG= membrane-associated proteins in eicosanoid and glutathione metabolism) proteins | subgroups I-IV | <25 | |
Protein Phosphotase 1 - A molecular mop for Microcystin
The initiator of fixing phosphate residues to proteins is an enzyme family called kinases. The adversaries of the phosphorylation process are the protein phosphatases. In general you classify them in three families: phosphoserine and phosphothreonine residues were dephosporylated by PPM and PPP families whereas PTP dehosphorylate phosphotyrosine amino acids. The role of regulation of cellular processes represent PP1 which is an ubiquitous eukaryotic enzyme belonging to the protein serine/threonine phosphatase class. The enormous variety and multifunctionality of PP1 concerning to cellular functions through the interaction of its catalytic subunit (PP1c) is written down in the publication "Functional diversity of protein phosphatase-1, a cellular economizer and reset button" http://www.ncbi.nlm.nih.gov/pubmed/14715909 Ceulemans et al.,2004. Next to wide diversity of biological function such as the regulation of blood-glucose levels and glycogen metabolism PP1 plays a major relevance in disease research which aims in the red biotechnology to produce new drug designs. The role of PP1 concerning to the HIV-1-transcription http://www.ncbi.nlm.nih.gov/pubmed/17266553 Nekhai et al.,2007 and Alzheimer disease brains http://www.ncbi.nlm.nih.gov/pubmed/8395566 Gong et al.,1993 illustrate this. Basically changes in the levels, phosphorylation status and conformation of the PP1c which consists of diverse regulatory subunits and domains hydrophobic grooves on the surface mostly through a short conserved binding motif RVxF leads to targeting of substrates and inhibitors. Together with the conserved structure figure 8 confirms the metalloenzyme characteristic of PP1: next to the potential substrate/inhibitor binding grooves with the C-Terminus at the end of the grooves a central β-α-β-α-β scaffold at the active site positions the two metal ions (Mn, Fe) which are coordinated by one asparagines, two aspartic acids and three histidines http://www.nature.com/nature/journal/v376/n6543/abs/376745a0.html Goldberg et al.,2002.An useable application of PP1 with regard to bioremediation implicates the iGEM project 2013 of team Dundee. PP1 as a natural binding protein accumulates the toxin microcystin(toxin released by Microcystis aeruginosato) combat the releases harm to mammals. Next to this microcystin plays also a role in the modulation of proliferation, apoptosis and proliferation of spermatogenic cells in vivo http://www.ncbi.nlm.nih.gov/pubmed/24025782 Zhou et al.,2013. The interaction of microcystin in form of the heptapeptide structure with three distinct regions of the PP1c shows figure 9. After converting the human PP1, given by iGEM Dundee, in RFC 25 and constructing some expression plasmids we produced transgenic phytomitrella patens (PF-14) with PP1 and a biobrick of PP1 receptor.
References:
- http://www.ncbi.nlm.nih.gov/pubmed/10051566 Beste et al., 1999 Beste G, Schmidt FS, Stibora T, Skerra A. (1999) Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold. PNAS, 96(5):1898-903.
- http://www.ncbi.nlm.nih.gov/pubmed/16307475 Vopel et al., 2005 Vopel S, Mühlbach H, Skerra A. (2005) Rational engineering of a fluorescein-binding anticalin for improved ligand affinity. Biol. Chem., 386(11):1097-104.
- http://www.ncbi.nlm.nih.gov/pubmed/9255793 Nord et al., 1997 Nord K, Gunneriusson E, Ringdahl J, Ståhl S, Uhlén M, Nygren PA. (1997) Binding proteins selected from combinatorial libraries of an α-helical bacterial receptor domain. Nature Biotech. 15(8):772-7.
- U.S. Geological Survey,2013
- http://www.ncbi.nlm.nih.gov/pubmed/15676296 Schlehuber and Skerra,2005 Schlehuber S, Skerra A.(2005)Lipocalins in drug discovery: from natural ligand-binding proteins to "anticalins".Drug Discov Today10(1):23-33
- http://www.pnas.org/content/103/15/6061 Yang et al.,2006 Yang Y.,Sulpice R.,Himmelbach A.,Meinhard M.,Christmann A., Grill E. Fibrillin expression is regulated by abscisic acid response regulators and is involved in abscisic acid-mediated photoprotection PNAS 6061–6066
- http://www.greenchicagoriver.com/ Homepage of the green chigargo river project
- [Homepage iGEM team Freiburg 2008]
- http://www.ncbi.nlm.nih.gov/pubmed/23792166 Scheib et al.,2007 Scheib U, Shanmugaratnam S, Farías-Rico JA, Höcker B.(2007)Change in protein-ligand specificity through binding pocket grafting J Struct Biol pii:S1047-8477(13)00157-3. doi: 10.1016/j.jsb.2013.06.002.
- http://www.pnas.org/content/96/5/1898.full.pdf Beste et al.,1999Beste G.,Schmidt F. S.,Stibora T.,Skerra A. (1999)Proc. Natl. Acad. Sci. USAVol. 96, pp. 1898 –1903
- http://onlinelibrary.wiley.com/doi/10.1002/prot.10497/abstract Korndörfer et. al. (2003)Korndörfer I.,Beste G.,Skerra A.(2003)Crystallographic analysis of an “anticalin” with tailored specificity for fluorescein reveals high structural plasticity of the lipocalin loop region Wileyvolume 53,issue 1,pages 121-129
- http://www.ncbi.nlm.nih.gov/pubmed/21428697 Oakley,2006 Oakley A.(2011)Drug Metab Rev43(2):138-51. doi: 10.3109/03602532.2011.558093
- http://www.ncbi.nlm.nih.gov/pubmed/16550164 McIlwain et al.,2006 McIlwain CC, Townsend DM, Tew KD(2006) Oncogene25(11):1639-48
- http://www.ncbi.nlm.nih.gov/pubmed/8770536 Hayes et al.,1995Hayes JD, Pulford DJ.(1995) The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance.Crit Rev Biochem Mol Biol.30(6):445-600
- http://www.ncbi.nlm.nih.gov/pubmed/20981235 Josephy, 2010 Josephy PD.(2010)Genetic variations in human glutathione transferase enzymes: significance for pharmacology and toxicology.Hum Genomics Proteomics 2010:876940. doi: 10.4061/2010/876940
- http://www.ncbi.nlm.nih.gov/pubmed/10971201 Hayes et al.,2000Hayes JD, Strange RC.(2000)Glutathione S-transferase polymorphisms and their biological consequencesPharmacology 61(3):154-66
- http://www.ncbi.nlm.nih.gov/pubmed/12563680 Fraser et al., 2003Fraser PA, Ding WZ, Mohseni M, Treadwell EL, Dooley MA, St Clair EW, Gilkeson GS, Cooper GS. (2003),J Rheumatol30(2):276-82
- http://www.sciencedirect.com/science/article/pii/0965174895000909 La-Aied Prapanthadara et al.,1996 La-Aied Prapanthadara,Surangchit Koottathe,Nongkran Promtet,Janet Hemingway†,Albert J., Ketterman (1996) Purification and characterization of a major glutathione S-transferase from the mosquito Anopheles dirus (Species B) Insect Biochemistry and Molecular BiologyVolume 26, Issue 3 Pages 277–285
- http://www.ncbi.nlm.nih.gov/pubmed/11038270 Kamencic et al.,2000 Kamencic H, Lyon A, Paterson PG, Juurlink BH.(2000)Monochlorobimane fluorometric method to measure tissue glutathione.Anal Biochem 286(1):35-7
- [Homepage iGEM team Caltech 2011]
- http://www.ncbi.nlm.nih.gov/pubmed/14715909 Ceulemans et al., 2004 Ceulemans H, Bollen M.(2004) Functional diversity of protein phosphatase-1, a cellular economizer and reset button.Physiol Rev.84(1):1-39.
- http://www.ncbi.nlm.nih.gov/pubmed/17266553 Nekhai et al.,2007 Nekhai S, Jerebtsova M, Jackson A, Southerland W.(2007)Regulation of HIV-1 transcription by protein phosphatase 1. Curr HIV Res. 5(1):3-9.
- http://www.ncbi.nlm.nih.gov/pubmed/8395566 Gong et al.,1993 Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K.(1993),J Neurochem61(3):921-7.
- http://www.nature.com/nature/journal/v376/n6543/abs/376745a0.html Goldberg et al.,2002 Jonathan Goldberg, Hsien-bin Huang, Young-guen Kwon, Paul Greengard, Angus C. Nairn and John Kuriyan (2002)Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1 Nature 376745 - 753 doi:10.1038/376745a0
- http://www.ncbi.nlm.nih.gov/pubmed/24025782 Zhou et al.,2013 Zhou Y, Chen Y, Yuan M, Xiang Z, Han X.(2013)J Toxicol Sci.38(5):661-70
- [Homepage iGEM team Dundee 2013]
- [[http://www.aerzteblatt.de/archiv/60535 Meißner M.(2008),Arzneimittel in der Umwelt: Natur als Medikamentendeponie,deutsches Ärzteblatt Artikel 64 S.20]]
- http://www.bfarm.de/SharedDocs/1_Downloads/EN/vigilance/csp/c-d/diclofenac.pdf;jsessionid=7EF043741A9EE95F1EEF8C14A116BBD7.1_cid332?__blob=publicationFile Core safty profil Diclofenac (2011)
- http://www.birdlife.org.uk/action/science/species/asia_vulture_crisis/index.html Article "Asia vulture crisis"
- http://www.nabu.de/tiereundpflanzen/voegel/international/03530.html Article "Katastrophales Geiersterben in Indien"
- http://www.sciencedirect.com/science/article/pii/S0269749111005173 Mohini et al., 2012 Mohini Sainia,Mark A. Taggartb,Dietmar Knopp,Suchitra Upretia,Devendra Swarupa,Asit Dasa,Praveen K. Guptaa,Reinhard Niessnerd,Vibhu Prakashf,Rafael Mateob,Richard J. Cuthbert (2012)Detecting diclofenac in livestock carcasses in India with an ELISA: A tool to prevent widespread vulture poisoning Environemntal pollution Elsevier volumen160 p.11-16
- http://www.ncbi.nlm.nih.gov/pubmed/20470236 Gan, 2010 Gan TJ.(2010) Diclofenac: an update on its mechanism of action and safety profile Curr Med Res Opin26(7):1715-31. doi: 10.1185/03007995.2010.486301
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1724805/ Predel et al.,2004 H Predel, R Koll, H Pabst, R Dieter, G Gallacchi, B Giannetti, M Bulitta, J Heidecker, and E Mueller(2004)Diclofenac patch for topical treatment of acute impact injuries: a randomised, double blind, placebo controlled, multicentre study Br J Sports Med 38(3): 318–323.
- http://www.ncbi.nlm.nih.gov/pubmed/11276273 Moragan et al.,2001 Morgan GJ Jr, Kaine J, DeLapp R, Palmer R (2001)Treatment of elderly patients with nabumetone or diclofenac: gastrointestinal safety profile J Clin Gastroenterol32(4):310-4.
- http://www.ncbi.nlm.nih.gov/pubmed/15480981 Chan et al.,2004 Chan FK, Hung LC, Suen BY, Wong VW, Hui AJ, Wu JC, Leung WK, Lee YT, To KF, Chung SC, Sung JJ.(2004) Celecoxib versus diclofenac plus omeprazole in high-risk arthritis patients: results of a randomized double-blind trial Gastroenterology 127(4):1038-43.
- http://www.ncbi.nlm.nih.gov/pubmed/22168216 Pavelka,2012 Pavelka K.(2012) A comparison of the therapeutic efficacy of diclofenac in osteoarthritis: a systematic review of randomised controlled trials Curr Med Res Opin.28(1):163-78. doi: 10.1185/03007995.2011.649848.
- http://www.ncbi.nlm.nih.gov/pubmed/11950570 Stoger et al.,2002 Stoger E, Sack M, Fischer R, Christou P.(2002) Plantibodies: applications, advantages and bottlenecks '#Curr Opin Biotechnol. 13(2):161-6.
- http://www.spiegel.de/wissenschaft/natur/insektengift-ddt-wie-die-malaria-wunderwaffe-bauern-in-die-armut-treibt-a-768654.html Koch L.,2011Koch L.(2011)Insektengift DDT: Wie die Malaria-Wunderwaffe Bauern in die Armut treibt Spiegel online
- http://www.ncbi.nlm.nih.gov/pubmed/11836138 Turusov et al.,2002 Turusov V, Rakitsky V, Tomatis L.(2002)Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risksEnviron Health Perspect.110(2):125-8
- http://www.ncbi.nlm.nih.gov/pubmed/16818570 Eskenazi et al.,2006 Eskenazi B, Marks AR, Bradman A, Fenster L, Johnson C, Barr DB, Jewell NP.(2006)In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children.Pediatrics118(1):233-41.
 "
"



AutoAnnotator:
Follow us:
Address:
iGEM Team TU-Munich
Emil-Erlenmeyer-Forum 5
85354 Freising, Germany
Email: igem@wzw.tum.de
Phone: +49 8161 71-4351