Team:TU-Munich/Project/Biodegradation
From 2013.igem.org
LouiseFunke (Talk | contribs) (→EreB) |
LouiseFunke (Talk | contribs) (→EreB) |
||
| Line 54: | Line 54: | ||
|colspan="7"| Table 1: | |colspan="7"| Table 1: | ||
====Antibiotics in WWTP effluents==== | ====Antibiotics in WWTP effluents==== | ||
| + | |- | ||
! align="center";| Antibiotic | ! align="center";| Antibiotic | ||
! align="center";| max. concentration [µg/l] | ! align="center";| max. concentration [µg/l] | ||
Revision as of 20:33, 15 September 2013
Biodegradation of Xenobiotics
Biodegradation is defined as "a process by which microbial organisms transform or alter the structure of chemicals introduced into the environment" (U.S. Environmental Protection Agency, 2009). We work with this concept by degrading or transforming noxious substances in waste water into non-hazardous compounds. These substances, such as antibiotics, hormones or pesticides cannot be removed by common waste water treatment plants, but it is possible to inactivate them with existing enzymes from natural catabolic mechanisms in different microorganisms. To utilize these enzymes we integrated them into a plant as a self-sustaining sedentary organism to create a functional water filter system. To illustrate this approach we chose three potent enzymes:
- The Erythromycin esterase, which degrades macrolides, a persistant group of antibiotics
- The Laccase BPUL, which degrades several noxious substances, for example the artificial hormone ethinyl estradiol, the main ingredient of contraception pills
- The Catechol-2,3-dioxygenase, catalyzing the degradation of aromatic pollutants, which occur in pesticides and insecticides
Erythromycin Esterase (EreB)
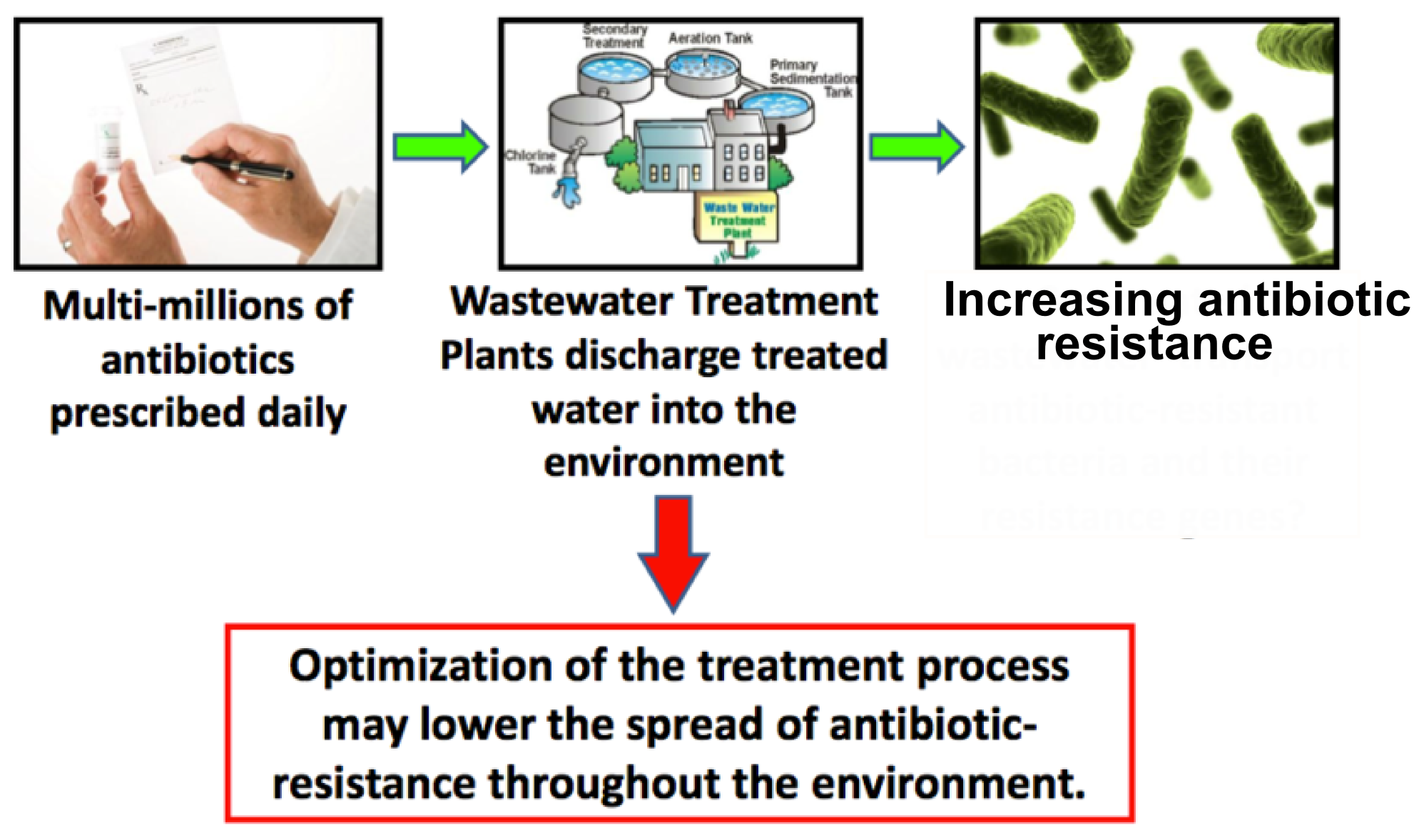
The Problem: Antibiotics in the environment
The discovery of antibiotics in the early 20th century has revolutionized the fight against infectious diseases. Due to their success thousands of tons of antibiotics have been produced, consumed and excreted, since antibiotics are designed to circulate through the body for a long time and therefore up to 75% leave the organism unaltered. As a result microbes are exposed to strong selective pressure to which these numerous and extremely adaptable organisms react by developing resistance mechanisms.
However, antimicrobial effectiveness is a precious, limited resource, that we can no longer do without. Accordingly, in 2000 a World Health Organization report focused on antibiotic resistance as one of the most critical human health challenges of the next century and called for “a global strategy to contain resistance”. In numbers, resistant pathogens affect 2 million Americans each year, of which 14,000 die as a result. In the EU antimicrobial resistance is responsible for 25,000 deaths every year and the annual treatment and social costs have been estimated at some €1.5 billion. These numbers will only rise if nothing is done to prevent precious antibiotics from becoming effectless.
Solutions
The obvious approach to minimize selective pressure on microorganisms is to deal with the problem of carefree, often precautionary overuse of antibiotics, not only in humans but also and especially in agriculture, by only using them when absolutely necessary.
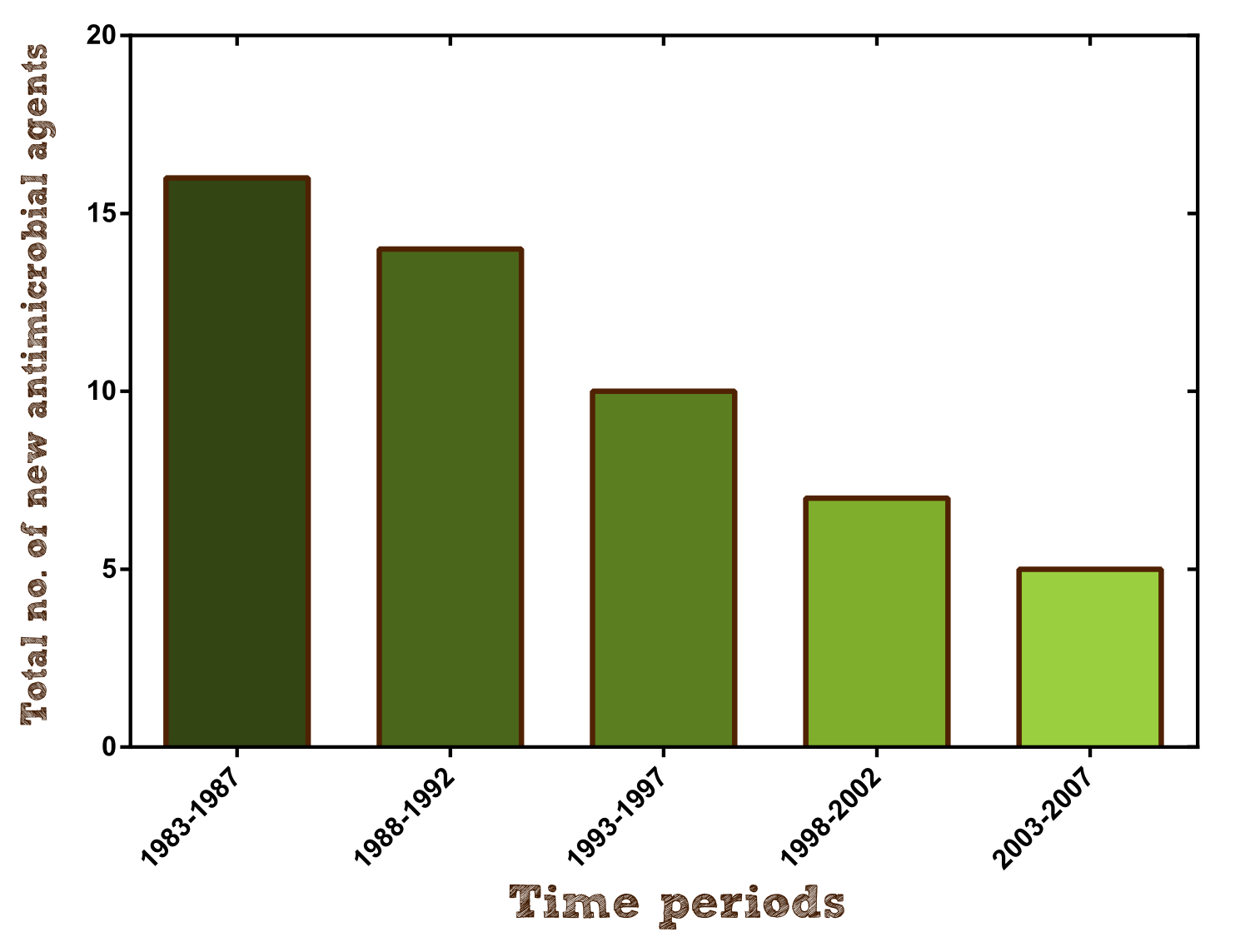
Furthermore research on new antibiotics should be hugely intensified to create alternative agents and keep pace with the formation of antimicrobial resistance. Unfortunately antibiotic development has slowed dramatically over the past 25 years because as a short-course and very effective therapy they have a much lower rate of return on investment than other drugs and therefore are financially not viable for pharmaceutical companies (per approved agent development costs are estimated to be $400-$800 million). For example in 2004 only 5 new antibacterial agents were under development by the largest pharmaceutical companies, barely beating the 4 new molecular entities to treat erectile dysfunction.
These two options are difficult to accomplish due to the increasing need of antibiotics with a growing world-wide population and the fact that finding new antibiotics is a costly, long-term and extremely difficult project. Therefore we focus on a third option to fight the spread of antibiotic resistance by developing a cheap, innovative water-filter that will prevent continuous inflow of the drugs into surface water and therefore reduce the selective pressure on microbes.
EreB
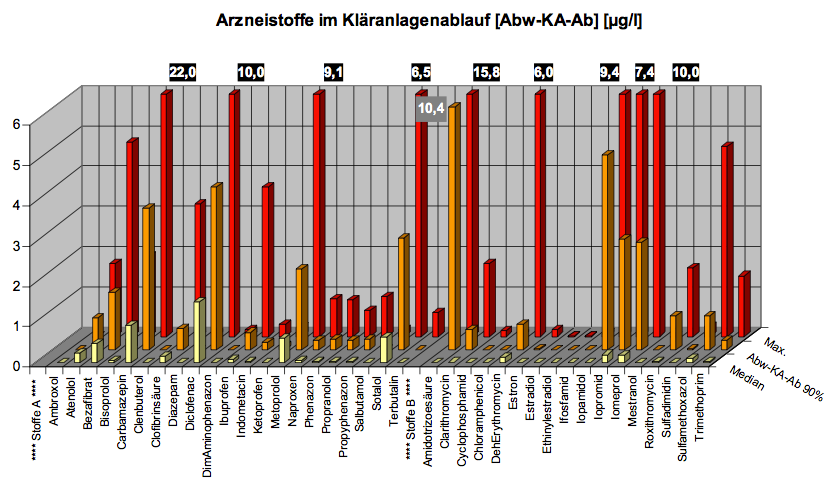
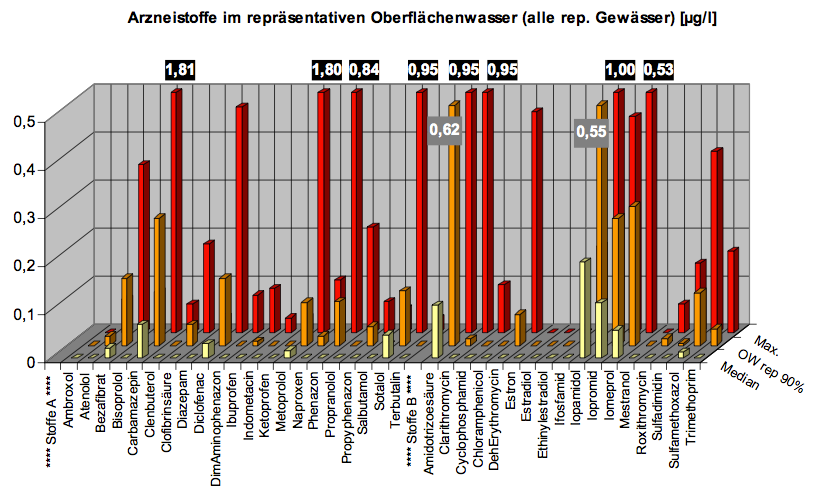
In our search for a suitable enzyme we looked at antibiotic concentrations in waste water. There have not yet been many investigations on this topic, one of the main surveys was executed by the German national committee for chemical safety (BLAC) of 202 effluent samples from 34 waste water treatment plants (WWTP). The result was that the main detectable antibiotics are the macrolides Erythromycin, Roxythromycin and Clarithromycin, the Sulfonamide Sulfamethoxalol as well as the Fluoroquinolone Ciprofloxacin. Most concentrations are about 100 ng/l, but maximum concentrations reached more than 3 µg/l. The often prescribed ß-lactam antibiotics, such as penicillin, were not detectable probably due to their fast degradation. Another important conclusion of the study is that the main source of antibiotic input into the aquatic environment is municipal waste water. Similar results were found in other countries (see table below).
Table 1:
Antibiotics in WWTP effluents | ||||||
| Antibiotic | max. concentration [µg/l] | min MIC50 [µg/l] | ||||
|---|---|---|---|---|---|---|
| Ciprofloxacin (fluoroquinolone) | 0.14 (D), 0.18 (USA), 0.74 (N) | 3 | ||||
| Clarithromycin (macrolide) | 1.00 (D), 0.30 (CH) | 2 | ||||
| Erythromycin (macrolide) | 1.10 (D), 0.17 (USA), 0.29 (CH), 2 (China) | 4 | ||||
| Roxythromycin (macrolide) | 1.00 (D), 0.28 (China) | 15 | ||||
| Sulfamethoxalol (sulfonamide) | 4.00 (D), 1.40 (USA) | 60 | ||||
| Trimethoprim (diaminopyrimidine) | 1.50 (D), 1.20 (USA), 1.30 (N) | 120 | ||||
Now we compared the concentrations of the most frequent antibiotics found in WWTP effluents to the minimum inhibitory concentration (MIC50) at which the growth of at least 50% of bacterial strains are inhibited. The MIC50 depends on the species. In the table the smallest MIC50 over all species is given, indicating the level at which the contamination seriously affects the microbiological system and therefore carrying out a certain stress which gives resistant individuals an increased selective advantage.
Our conclusion from these data was that macrolides are a hazardous antibiotic class, from which several representatives are found in WWTP effluents and surface water in concentrations relatively close to their MIC50. Macrolides are important and clinically relevant agents used to treat infectious diseases, first introduced into clinics in 1952 as an alternative to penicillin. Today they are mainly applied for treatment of respiratory diseases, including pneumonia, the leading cause of childhood deaths worldwide. The drug functions by inhibiting protein biosynthesis when it binds to the exit tunnel of the 50S ribosomal subunit which leads to abortion of the growth of nascent peptide chains.
Macrolides can be enzymatically inactivated with the macrolide esterases EreA and EreB, from which we chose EreB as our model enzyme since it has a broader degradation spectrum than EreA and is not metal-dependent.
EreB is a cytoplasmic protein that belongs to the hydrolase superfamily catalyzing the opening of the macrolactone ring, that is characteristic for macrolides and was originally discovered in E.Coli on an exogeneous plasmid. After the enzymatic opening of this cyclic ester bond a non-enzymatic hemiketal formation takes place followed by condensation and dehydration. The only co-substrate needed by the enzyme for hydrolysis is water which also makes it a suitable candidate for our moss-filter.
More information on the enzyme can be found for example in a very detailed recently published paper by Wright et al., who also generously provided us with the plasmid DNA.
Laccase (Bacillus pumilus)
General Information
Laccases are implicated in a wide spectrum of biological activities and, in particular, play a key role in morphogenesis, development and lignin metabolism in fungi, bacteria and plants. For example, they are necessary to degrade lignin in Basidiomycetes and synthesize complex polymers like Melanin in Ascomycentes. Laccases belong to the enzyme class of Oxidoreductases (EC 1.10.3.2). They are also known as monophenolic Oxidases, because of their substrate specifity.It is widely believed that Laccases are the simpliest representatives of the ubiquitous blue multi-copper oxidase family. Laccases are extracellular enzymes consiststing usually of 15-20 % carbon-hydrogen. Their molecular weight ranges deglycated from 60 to 80 kDa. Further on Laccases can occur as monomers, dimers, trimers and tetramers.
Active site and catalyzed reaction
The specific blue copper ions in the active site of Laccases are essential for the enzyme mediated radical oxidation of phenolic groups. In general, Laccases catalyze the linked oxidation of many aromatic and phenolic substances while the phenolic group is oxidized to a radical and oxygen is reduced to a hydrate. The active site of the enzyme includes a four-copper-ion-cluster which can be detected by using Spectroscopy. The cluster consists of one type 1 blue copper-ion, one type 2 and two type 3 copper-ions. In this reaction the electron from the oxidation is transfered to the other three copper ions. These ions form a trinuclearic cluster, which transfers electrons to the terminal electron acceptor oxygen. In the end, molecular oxygen is reduced, by receiving four electrons, to water.
Possible applications of Laccases
Due to their ability to oxidize phenolic and nonphenolic lignin related compounds, as well as highly recalcitrant environmental pollutants, Laccases possess a high potential as a bioremediation agent in synthetic biology. Waste water from hospitals, textile and paper industry, as well as in sewage treatment plants can be detoxified. Possible substrates may be natural and synthetic Estrogens, Xenoestrogens and polycylic aromatic hydrocarbons. Laccases may also be used to degrade high polymeric Lignin to make use of the polymer for energy generation. Carcinogenic, mutagenic aromatic hydrocarbons and estrogens, xenoestrogens and polycyclic aromatic hydrocarbons can be oxidized and detoxified by laccases, too. In addition to that, Laccases can oxidize up herbicides, pesticides in soil.
Laccase BPUL from Bacillus Pumilus BBa_K863000
In this project, we used the BPUL Laccase from Bacillus Pumilus BBa_K863000, which was established and well characterized by the iGEM Team of Bielefeld in 2012 . The Laccase, which was prepared and sent to the Parts Registry by the team of Bielefeld was fused to a Histag and a Ribosome Binding Site. The molecular weight of this Laccase is 58.6 kDa. Comparing the enzyme activity between 25°C and 10°C, you can see that it is influenced marginal, but it slightly decreases. Its pH optimum is reached at pH 4 to 5. The differences in activity varying the concentration of CuCl2, differ minimal. An optimum is reached at 0.6 mM. An increase of the Concentration of CuCl2 decreases the enzyme activity of the Laccase. The lower the used concentration of MeOH and Acetonitrile was, the higher the enzyme activity was detected. Bielefeld established many different Laccases in iGEM 2012, but we chose this one, because it seemed to be the most effective one, they have characterized.
Catechol Dioxygenase (XylE)
As ever more third world countries around the globe experience a rapid industrialization without establishing proper environmental practices as well as big companies outsourcing their production facilities in order to avoid expensive sewage treatment the problem of water pollution is more urgent then ever. In order to tackle this problem we were looking for a suitable pollutant which our moss could degrade resulting in a better overall water quality. The organic compund catechol, or 1,2-dihydroxybenzene, met all our criteria as it was a byproduct or raw material in several processes in the chemical industry as well as a stable product of several natural pathways in microbia degrading catechol-like pollutants. Further research into this topic showed that catechol was already a popular target for degredation not only in the iGEM competition but also regarding phytoremediation were it was degraded by Arabidopsis thaliana. Nevertheless we felt that no applicable or effective method to remove catechol from the environment was presented yet, as microbial degredation is unsuitable to establish in poor countries as to its lower cost-effectiveness compared to our moss system. Additionally a removal of the highly soluble catechol through a waterborne moss like Physcomitrella patens seems more fitting then through other plants.
Catechol
Pollution
References:
- [Mayer AM, Staples RC, 2002] Mayer AM, Staples RC (2002). Laccase: new functions for an old enzyme. Phytochemistry, 60(6):551-65.
- [Claus H.2004] Claus H. (2004). Laccases: structure, reactions, distribution. Micron., 2004;35(1-2):93-6.
- [Madhavi and Lele (2009)] Madhavi and Lele (2009). Laccase properties, use. Bioresources, 4(4), 1694-1717.
 "
"





AutoAnnotator:
Follow us:
Address:
iGEM Team TU-Munich
Emil-Erlenmeyer-Forum 5
85354 Freising, Germany
Email: igem@wzw.tum.de
Phone: +49 8161 71-4351