Team:Paris Saclay/Notebook/July/16
From 2013.igem.org
(Difference between revisions)
| Line 83: | Line 83: | ||
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" |[[File:Psgel21607.jpg| | + | | style="width:350px;border:1px solid black;" |[[File:Psgel21607.jpg|300px]] |
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
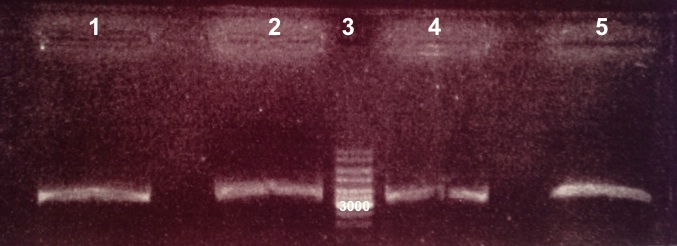
* Well 1 to 5 : 2µL Bba_K1155001 digested by EcoRI/PstI+2µl of 6X loading dye | * Well 1 to 5 : 2µL Bba_K1155001 digested by EcoRI/PstI+2µl of 6X loading dye | ||
Revision as of 23:47, 28 September 2013
Notebook : July 16
Lab work
A - Aerobic/Anaerobic regulation system
Objective : obtaining Bba_K1155003, Bba_K1155007
1 - Extraction of Bba_B0015, Bba_B0017, Bba_I732019 from DH5α
Zhou
Protocol : High copy plamid extraction
2 - Digestion of Bba_B0015, Bba_B0017, Bba_I732019 by EcoRI/PstI
Anaïs
- DNA : 5µL
- Buffer FD : 2µL
- EcoRI FD : 1µL
- PstI FD : 1µL
- H2O : 21µL
We let our digestion 1h30 at 37°C.
Objective : obtaining biobricks in PSB3K3
1 - Digestion of PSB3K3 by EcoRI/PstI
Anaïs
Used quantities :
- DNA : 5µL
- Buffer FD : 2µL
- EcoRI FD : 1µL
- PstI FD : 1µL
- H2O : 11µL
We let our digestion 1h30 at 37°C.
2 - Electrophoresis of the digestion of PSB3K3 by EcoRI/PstI
Sheng
Expected sizes :
- PSB3K3 : 2750bp
|
We obtain fragments at the right size. |
3 - Gel purification of electrophoresis of the digestion of PSB3K3 by EcoRI/PstI
Abdou
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
B - PCB sensor system
Objective : obtaining Bba_K1155001, Bba_K1155002, BphR2 in PSB1C3
1 - Electrophoresis of the digestion of Bba_K1155001, Bba_K1155002, BphR2 in PSB1C3
Anaïs, Zhou
- Bba_K1155001 :

|
|
Expected size :
- Bba_K1155001 digested by EcoRI/PstI : 2037bp + 333bp
- Bba_K1155001 digested by SacII : 2370bp
|
We obtain fragment at the right size. We will amplify Bba_K1155001. |
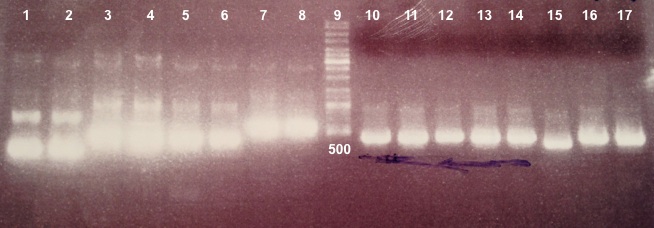
- Bba_K1155002 :
|
|
Expected size :
- Bba_K1155002 digested by EcoRI/PstI : ...
- Bba_K1155002 digested by SacII : ...
|
We didn't obtain fragments at the right size. We will do the ligation again. |
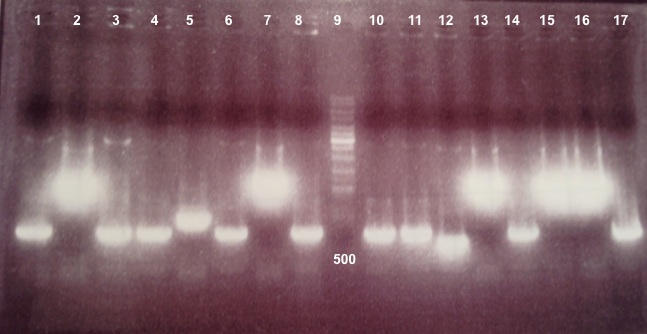
- BphR2 in PSB1C3 :
|
|
Expected size :
- BphR2 digested by EcoRI/PstI : ...
- BphR2 digested by SacII : ...
|
We didn't obtain fragments at the right size. After reading the sequence again, we find an digestion site in the middle of our biobrick. We will do a Gibson assembly to modify this site. |
| Previous day | Back to calendar | Next day |
 "
"