Team:Leeds
From 2013.igem.org
(Difference between revisions)
| Line 27: | Line 27: | ||
We foresee Micro-Beagle being adapted for both the detection of waterborne pathogens and a variety of other diagnostic applications, and we envision future multisensor Micro-Beagles in which diverse pathogens can be simultaneously and quantitatively measured from a single water sample | We foresee Micro-Beagle being adapted for both the detection of waterborne pathogens and a variety of other diagnostic applications, and we envision future multisensor Micro-Beagles in which diverse pathogens can be simultaneously and quantitatively measured from a single water sample | ||
<br> | <br> | ||
| - | |||
| + | <span style="color: red"> | ||
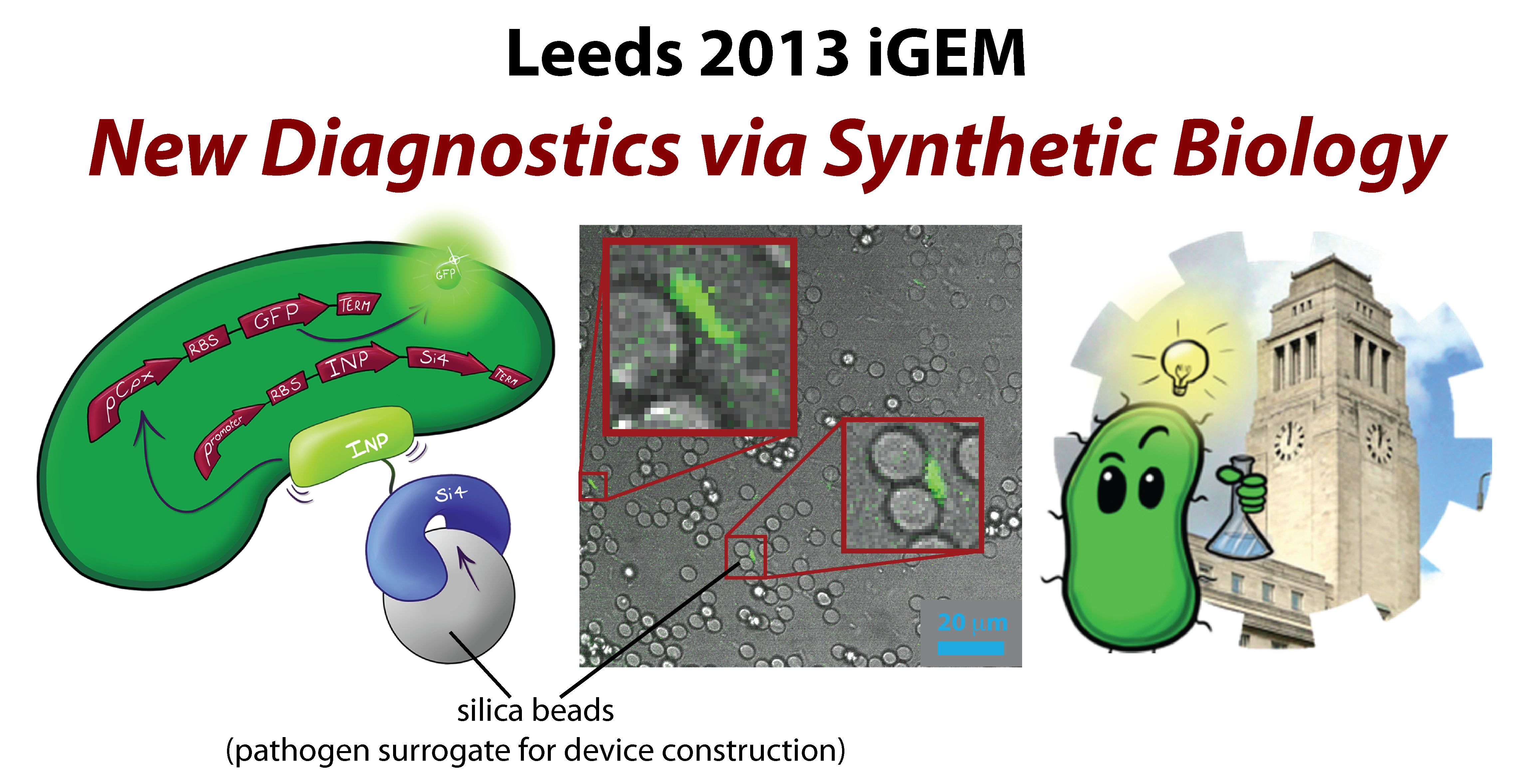
Like the Beagle hunting dog chasing hares, our Micro-beagle is a biosensor designed to hunt down pathogens. Micro-beagle utilises the Cpx pathway and GFP, which, when it fluoresces can be seen with the naked eye. | Like the Beagle hunting dog chasing hares, our Micro-beagle is a biosensor designed to hunt down pathogens. Micro-beagle utilises the Cpx pathway and GFP, which, when it fluoresces can be seen with the naked eye. | ||
<br> | <br> | ||
| - | + | <span style="color: red"> | |
In order to achieve this we will have designed our biosensor to physically bind to antigens or particles. This binding will induce membrane stress activating the Cpx pathway. By placing the GFP gene downstream of the Cpx promoter, GFP protein will be produced following membrane stress consequently causing the cells tol fluoresce when the pathogen is present in the solution. | In order to achieve this we will have designed our biosensor to physically bind to antigens or particles. This binding will induce membrane stress activating the Cpx pathway. By placing the GFP gene downstream of the Cpx promoter, GFP protein will be produced following membrane stress consequently causing the cells tol fluoresce when the pathogen is present in the solution. | ||
<br> | <br> | ||
| - | + | <span style="color: red"> | |
As a proof of concept, we will be using silica beads as target analogue for pathogens. In order to bind to the beads will be expressing a silica binding peptide on the surface of the cells using Ice Nucleation Protein (INP). INP allows expression of any gene placed at its C terminus on the cell surface. The theory is that the silica binding peptide sequence can be easily swapped for a sequence of any antigen binding moiety and therefore enable us to detect any pathogen simply, quickly and cheaply. | As a proof of concept, we will be using silica beads as target analogue for pathogens. In order to bind to the beads will be expressing a silica binding peptide on the surface of the cells using Ice Nucleation Protein (INP). INP allows expression of any gene placed at its C terminus on the cell surface. The theory is that the silica binding peptide sequence can be easily swapped for a sequence of any antigen binding moiety and therefore enable us to detect any pathogen simply, quickly and cheaply. | ||
<br> | <br> | ||
| - | + | <span style="color: red"> | |
Upon completion of our project our suggested initial use for the Micro-Beagle will be a low cost easy to use test used to assess the efficacy of water treatment systems. However as stated earlier the detection system can easily be modified to allow any other large particles whose identification could be of value | Upon completion of our project our suggested initial use for the Micro-Beagle will be a low cost easy to use test used to assess the efficacy of water treatment systems. However as stated earlier the detection system can easily be modified to allow any other large particles whose identification could be of value | ||
| + | </span> | ||
<br> | <br> | ||
==Who the heck are Leeds?== | ==Who the heck are Leeds?== | ||
Revision as of 15:33, 30 September 2013
</div>
 "
"