Team:Paris Saclay/Notebook/July/26
From 2013.igem.org
(→2 - Electrophoresis of the digestion of PCR products : RBS-AmilCP) |
|||
| (24 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
==='''A - Aerobic/Anaerobic regulation system'''=== | ==='''A - Aerobic/Anaerobic regulation system'''=== | ||
| - | ===='''Objective : obtaining | + | ===='''Objective : obtaining BBa_K1155003'''==== |
| - | ===='''1 - Ligation of RBS-AmilCP and Term- | + | ===='''1 - Inactivation of EcoRI/SpeI used for the digestion of PCR products : RBS-AmilCP '''==== |
| + | |||
| + | Abdou | ||
| + | |||
| + | Protocol : [[Team:Paris_Saclay/ethanol|Ethanol precipitation]] | ||
| + | |||
| + | We used 90µL of DNA. | ||
| + | At the end, we mix our DNA with 20µL of water. | ||
| + | |||
| + | ===='''2 - Electrophoresis of the digestion of PCR products : RBS-AmilCP'''==== | ||
| + | |||
| + | Abdou | ||
| + | |||
| + | {| | ||
| + | | style="width:350px;border:1px solid black;" |[[File:Psgel2607.jpg]] | ||
| + | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| + | * Well 1 : 5µL of RBS-AmilCP+1µL of 6X loading dye | ||
| + | * Well 2 : 6µL of DNA Ladder | ||
| + | * Gel : 1% | ||
| + | |} | ||
| + | |||
| + | Expected sizes : | ||
| + | * RBS-AmilCP : 3500bp | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We obtain fragments at the right size. We will ligate RBS-AmilCP with Term. | ||
| + | |} | ||
| + | |||
| + | ===='''3 - Ligation of RBS-AmilCP and Term-pSB1C3'''==== | ||
Xavier | Xavier | ||
| Line 16: | Line 45: | ||
* RBS-AmilCP : 2µL | * RBS-AmilCP : 2µL | ||
| - | * | + | * Term in pSB1C3 : 2.5µL |
* Buffer : 1µL | * Buffer : 1µL | ||
* Ligase : 1µL | * Ligase : 1µL | ||
* H2O : 3.5µL | * H2O : 3.5µL | ||
| - | ===='''Objective : obtaining | + | ===='''Objective : obtaining BBa_K1155004, BBa_K1155005, BBa_K1155006'''==== |
===='''1 - Electrophoresis of PCR products : NarK, NarG, NirB'''==== | ===='''1 - Electrophoresis of PCR products : NarK, NarG, NirB'''==== | ||
| Line 28: | Line 57: | ||
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" |[[]] | + | | style="width:350px;border:1px solid black;" |[[File:Psgel12607.jpg]] |
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
* Well 1 : 5µL of NirB+1µL of 6X loading dye | * Well 1 : 5µL of NirB+1µL of 6X loading dye | ||
| - | * Well 2 : 5µL of NirB+1µL of 6X loading dye | + | * Well 2 : 5µL of NirB+1µL of 6X loading dye |
* Well 3 : 5µL of NarG+1µL of 6X loading dye | * Well 3 : 5µL of NarG+1µL of 6X loading dye | ||
* Well 4 : 5µL of NarG+1µL of 6X loading dye | * Well 4 : 5µL of NarG+1µL of 6X loading dye | ||
* Well 5 : 6µL DNA Ladder | * Well 5 : 6µL DNA Ladder | ||
| - | * Well 6 : 5µL of NarK+1µL of 6X loading dye | + | * Well 6 : 5µL of NarK+1µL of 6X loading dye |
* Well 7 : 5µL of NarK+1µL of 6X loading dye | * Well 7 : 5µL of NarK+1µL of 6X loading dye | ||
* Well 8 : 5µL of NarK+1µL of 6X loading dye | * Well 8 : 5µL of NarK+1µL of 6X loading dye | ||
| Line 42: | Line 71: | ||
Expected sizes : | Expected sizes : | ||
| - | * NarK, NarG, NirB : | + | * NarK, NarG, NirB : 200bp |
{| | {| | ||
| Line 53: | Line 82: | ||
Abdou, Xavier | Abdou, Xavier | ||
| - | Used quantities : | + | Used quantities : |
| - | ... | + | |
| + | * NarK : | ||
| + | ** Buffer phusion : 10µL | ||
| + | ** dNTP : 1µL | ||
| + | ** Oligo 47 : 2µL | ||
| + | ** Oligo 48 : 2µL | ||
| + | ** Concentrated DNA : 2µL | ||
| + | ** Phusion : 0.25µL | ||
| + | |||
| + | * NarG : | ||
| + | ** Buffer phusion : 10µL | ||
| + | ** dNTP : 1µL | ||
| + | ** Oligo 49 : 2µL | ||
| + | ** Oligo 50 : 2µL | ||
| + | ** Concentrated DNA : 2µL | ||
| + | ** Phusion : 0.25µL | ||
| + | |||
| + | * NirB : | ||
| + | ** Buffer phusion : 10µL | ||
| + | ** dNTP : 1µL | ||
| + | ** Oligo 45 : 2µL | ||
| + | ** Oligo 46 : 2µL | ||
| + | ** Concentrated DNA : 2µL | ||
| + | ** Phusion : 0.25µL | ||
| + | |||
| + | PCR Program : | ||
| - | + | [[File:PsPCR2507.jpg|400px]] | |
| - | + | ||
===='''3 - Electrophoresis of PCR products : NarK, NarG, NirB'''==== | ===='''3 - Electrophoresis of PCR products : NarK, NarG, NirB'''==== | ||
| Line 64: | Line 117: | ||
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" |[[]] | + | | style="width:350px;border:1px solid black;" |[[File:Psgel22607.jpg]] |
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| - | * Well 1 : 2µL of NirB+ | + | * Well 1 : 2µL of NirB+3µL H2O+1µL of 6X loading dye |
| - | * Well 2 : 2µL of NirB+1µL of 6X loading dye | + | * Well 2 : 2µL of NirB+3µL H2O+1µL of 6X loading dye |
| - | * Well 3 : 5µL of NarK+1µL of 6X loading dye | + | * Well 3 : 5µL of NarK+3µL H2O+1µL of 6X loading dye |
| - | * Well 4 : 5µL of NarK+1µL of 6X loading dye | + | * Well 4 : 5µL of NarK+3µL H2O+1µL of 6X loading dye |
* Well 5 : 6µL DNA Ladder | * Well 5 : 6µL DNA Ladder | ||
| - | * Well 6 : 5µL of NarG+1µL of 6X loading dye | + | * Well 6 : 5µL of NarG+3µL H2O+1µL of 6X loading dye |
| - | * Well 7 : 5µL of NarG+1µL of 6X loading dye | + | * Well 7 : 5µL of NarG+3µL H2O+1µL of 6X loading dye |
* Gel : 1.2% | * Gel : 1.2% | ||
|} | |} | ||
Expected sizes : | Expected sizes : | ||
| - | * NarK, NarG, NirB : | + | * NarK, NarG, NirB : 200bp |
{| | {| | ||
| Line 88: | Line 141: | ||
Xavier | Xavier | ||
| - | Protocol : [ | + | Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ] |
| - | + | At the end we mix our DNA in 20µL of H2O. | |
| + | ===='''5 - Digestion of PCR products : NarK, NarG, NirB by EcoRI/PstI'''==== | ||
| + | |||
| + | Abdou | ||
| + | |||
| + | Quantities used : | ||
| + | |||
| + | * NarK, NarG, NirB : 10µL | ||
| + | * Buffer FD : 3µL | ||
| + | * EcoRI FD : 1.5µL | ||
| + | * PstI FD : 1.5µL | ||
| + | * H2O : 14µL | ||
| + | |||
| + | We let the digestion at 37°C during 1h30 and then at -20°C. | ||
| + | |||
| + | |||
| + | ==='''B - PCB sensor system'''=== | ||
| + | |||
| + | ===='''Objective : obtaining BBa_K1155002'''==== | ||
| + | |||
| + | ===='''1 - Tranformation of BBa_K1155002 in DH5α '''==== | ||
| + | |||
| + | Abdou, Xavier | ||
| + | |||
| + | Protocol : [[Team:Paris_Saclay/Protocols/Transformation|Bacterial tranformation]] | ||
| + | |||
| + | {| border="1" align="center" | ||
| + | |[[Team:Paris Saclay/Notebook/July/25|<big>Previous day</big>]] | ||
| + | |||
| + | |[[Team:Paris_Saclay/Notebook|<big>Back to calendar</big>]] | ||
| + | |||
| + | |[[Team:Paris Saclay/Notebook/July/30|<big>Next day</big>]] | ||
| + | |} | ||
{{Team:Paris_Saclay/incl_fin}} | {{Team:Paris_Saclay/incl_fin}} | ||
Latest revision as of 00:29, 5 October 2013
Notebook : July 26
Lab work
A - Aerobic/Anaerobic regulation system
Objective : obtaining BBa_K1155003
1 - Inactivation of EcoRI/SpeI used for the digestion of PCR products : RBS-AmilCP
Abdou
Protocol : Ethanol precipitation
We used 90µL of DNA. At the end, we mix our DNA with 20µL of water.
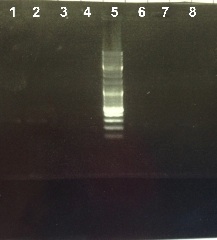
2 - Electrophoresis of the digestion of PCR products : RBS-AmilCP
Abdou

|
|
Expected sizes :
- RBS-AmilCP : 3500bp
|
We obtain fragments at the right size. We will ligate RBS-AmilCP with Term. |
3 - Ligation of RBS-AmilCP and Term-pSB1C3
Xavier
Used quantities :
- RBS-AmilCP : 2µL
- Term in pSB1C3 : 2.5µL
- Buffer : 1µL
- Ligase : 1µL
- H2O : 3.5µL
Objective : obtaining BBa_K1155004, BBa_K1155005, BBa_K1155006
1 - Electrophoresis of PCR products : NarK, NarG, NirB
Abdou
Expected sizes :
- NarK, NarG, NirB : 200bp
|
We lost all our PCR products. We will do PCR again using more concentrated DNA and oligos. |
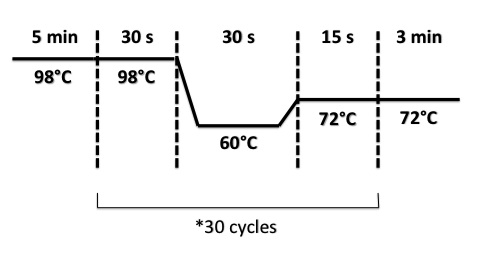
2 - PCR of NarK, NarG, NirB
Abdou, Xavier
Used quantities :
- NarK :
- Buffer phusion : 10µL
- dNTP : 1µL
- Oligo 47 : 2µL
- Oligo 48 : 2µL
- Concentrated DNA : 2µL
- Phusion : 0.25µL
- NarG :
- Buffer phusion : 10µL
- dNTP : 1µL
- Oligo 49 : 2µL
- Oligo 50 : 2µL
- Concentrated DNA : 2µL
- Phusion : 0.25µL
- NirB :
- Buffer phusion : 10µL
- dNTP : 1µL
- Oligo 45 : 2µL
- Oligo 46 : 2µL
- Concentrated DNA : 2µL
- Phusion : 0.25µL
PCR Program :
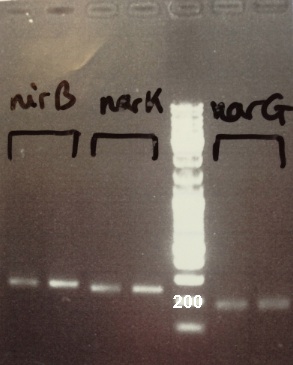
3 - Electrophoresis of PCR products : NarK, NarG, NirB
Abdou, Xavier
Expected sizes :
- NarK, NarG, NirB : 200bp
|
We obtain fragments at the right size. We can purify it. |
4 - Gel purification of PCR products : NarK, NarG, NirB
Xavier
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
At the end we mix our DNA in 20µL of H2O.
5 - Digestion of PCR products : NarK, NarG, NirB by EcoRI/PstI
Abdou
Quantities used :
- NarK, NarG, NirB : 10µL
- Buffer FD : 3µL
- EcoRI FD : 1.5µL
- PstI FD : 1.5µL
- H2O : 14µL
We let the digestion at 37°C during 1h30 and then at -20°C.
B - PCB sensor system
Objective : obtaining BBa_K1155002
1 - Tranformation of BBa_K1155002 in DH5α
Abdou, Xavier
Protocol : Bacterial tranformation
| Previous day | Back to calendar | Next day |
 "
"