Team:NYMU-Taipei/Project/Kill
From 2013.igem.org
| (16 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
===Background=== | ===Background=== | ||
| - | *Ethanol | + | *Ethanol is known as a kind of toxins to bees. Up to certain degree, it has potential harm to the nervous system of bees. Besides, it could have influence on hydrostatical pressure, which is one of essential factors to the germination of'' Nosema ceranae''. |
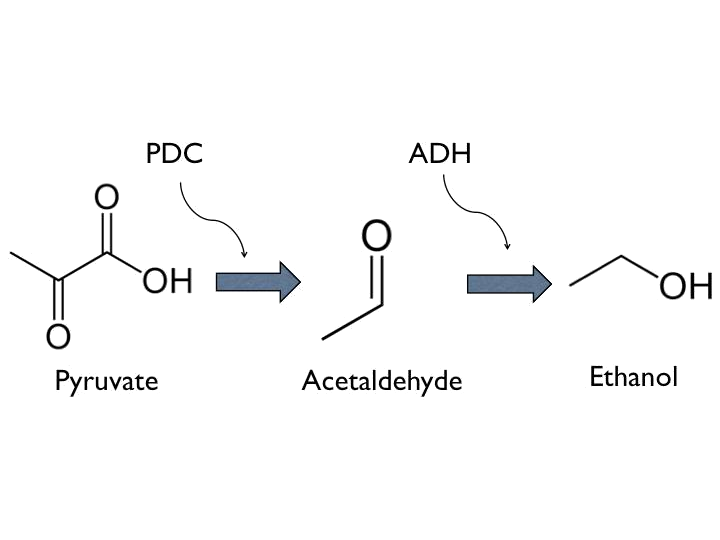
*Ethanol can be produced from pyruvate through [http://parts.igem.org/Part:BBa_K1104400 Pyruvate Decarboxylase (PDC)] and [http://parts.igem.org/Part:BBa_K1104401 Alcohol Dehydrogenase (ADH)]. | *Ethanol can be produced from pyruvate through [http://parts.igem.org/Part:BBa_K1104400 Pyruvate Decarboxylase (PDC)] and [http://parts.igem.org/Part:BBa_K1104401 Alcohol Dehydrogenase (ADH)]. | ||
*Cry toxins have specific activities against insect species of the orders Lepidoptera (moths and butterflies), Diptera (flies and mosquitoes), Coleoptera (beetles), Hymenoptera (wasps, bees, ants and sawflies) and nematodes. | *Cry toxins have specific activities against insect species of the orders Lepidoptera (moths and butterflies), Diptera (flies and mosquitoes), Coleoptera (beetles), Hymenoptera (wasps, bees, ants and sawflies) and nematodes. | ||
*When insects ingest toxin crystals, the alkaline pH of their digestive tract denatures the insoluble crystals, making them soluble and thus amenable to being cut with proteases found in the insect gut, which liberate the cry toxin from the crystal. The Cry toxin is then inserted into the insect gut cell membrane, paralyzing the digestive tract and forming a pore. The insect stops eating and starves to death. | *When insects ingest toxin crystals, the alkaline pH of their digestive tract denatures the insoluble crystals, making them soluble and thus amenable to being cut with proteases found in the insect gut, which liberate the cry toxin from the crystal. The Cry toxin is then inserted into the insect gut cell membrane, paralyzing the digestive tract and forming a pore. The insect stops eating and starves to death. | ||
| - | === | + | ===Methods and Circuit design=== |
| - | ==== | + | ====Methods==== |
The infected honeybee can go back to their hive, contact other bees, and spread the pathogen in a very short time. To prevent the whole bees in hive from infected,we come up with a way to prohibit ''Nosema'' from speading. The method is composed of two parts. | The infected honeybee can go back to their hive, contact other bees, and spread the pathogen in a very short time. To prevent the whole bees in hive from infected,we come up with a way to prohibit ''Nosema'' from speading. The method is composed of two parts. | ||
*1. How to post-sensing if'' Bee. coli'' could not successfully kill the ''Nosema''. | *1. How to post-sensing if'' Bee. coli'' could not successfully kill the ''Nosema''. | ||
| Line 23: | Line 23: | ||
====Circuit design==== | ====Circuit design==== | ||
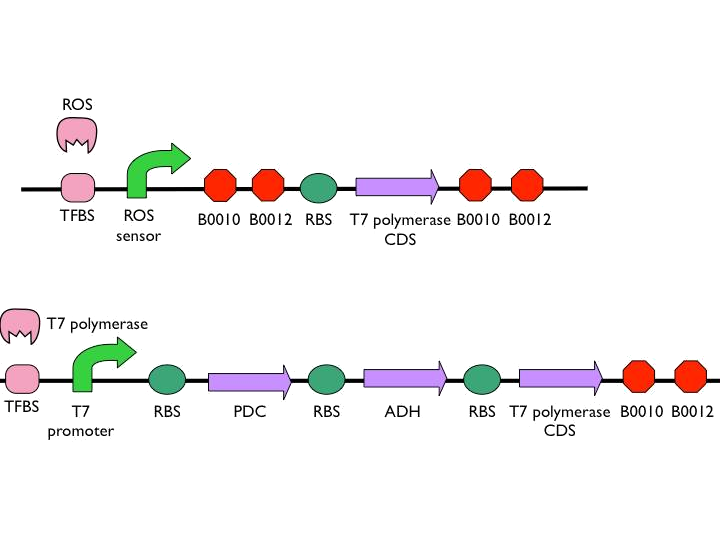
| - | *According to the result of modeling, it is obvious that we should put 1~2 terminators whose forward efficiency is about 0.98 CC after our hybrid promoter. The purpose of the terminators is to prevent bees from dying easily.The purpose of the terminators is to prevent bees from dying easily. On top of that, the other important part is the positive feedback circuit. It is useless that we only produce very few pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) | + | *According to the result of modeling, it is obvious that we should put 1~2 terminators whose forward efficiency is about 0.98 CC after our hybrid promoter. The purpose of the terminators is to prevent bees from dying easily.The purpose of the terminators is to prevent bees from dying easily. On top of that, the other important part is the positive feedback circuit. It is useless that we only produce very few pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) or Cry toxin. Hence we introduced some biobricks to our circuit to exert efficiency. |
[[image:NYMU_NO7_ethanol-producing.png]] | [[image:NYMU_NO7_ethanol-producing.png]] | ||
*The coding sequence of the first train is [http://parts.igem.org/Part:BBa_K145001 Part:BBa_K145001], which is the upstream DNA sequence of T7 polymerase. Otherwise, one of the coding sequence on the second strain will also be transcribed into the mRNA of T7 polymerase. In order to construct positive feedback of the alcohol-producing enzymes, we set a T7 polymerase coding sequence on the second train. Meanwhile in front of the second train, we place the receptor of T7 polymerase and [http://parts.igem.org/Part:BBa_I712074 T7 promoter]. In case of the leaky condition, we choose T7 polymerase as our go-between of alcohol-producing enzymes proliferating system. | *The coding sequence of the first train is [http://parts.igem.org/Part:BBa_K145001 Part:BBa_K145001], which is the upstream DNA sequence of T7 polymerase. Otherwise, one of the coding sequence on the second strain will also be transcribed into the mRNA of T7 polymerase. In order to construct positive feedback of the alcohol-producing enzymes, we set a T7 polymerase coding sequence on the second train. Meanwhile in front of the second train, we place the receptor of T7 polymerase and [http://parts.igem.org/Part:BBa_I712074 T7 promoter]. In case of the leaky condition, we choose T7 polymerase as our go-between of alcohol-producing enzymes proliferating system. | ||
| - | [[File:NYMU_no7_circuit.png|850px|''' Suicide Circuit''']] | + | [[File:NYMU_no7_circuit.png|850px|''' Suicide Circuit with EtOH''']] |
| + | [[File:Nymu_no.7_crytoxin.png|850px|''' Suicide Circuit with Cry toxin''']] | ||
| + | |||
==<font color=darkorange>'''Experiment'''</font>== | ==<font color=darkorange>'''Experiment'''</font>== | ||
===Test the producing efficiency with positive feedback=== | ===Test the producing efficiency with positive feedback=== | ||
| Line 34: | Line 36: | ||
#According to the experiment made by 5th group, we could know the efficiency of ROS promoter | #According to the experiment made by 5th group, we could know the efficiency of ROS promoter | ||
#According to the experiment made by Caitlin Conboy, we could know the forward efficiency of B0015[http://parts.igem.org/Help:Terminators/Measurement/Caitlin_Conboy] | #According to the experiment made by Caitlin Conboy, we could know the forward efficiency of B0015[http://parts.igem.org/Help:Terminators/Measurement/Caitlin_Conboy] | ||
| - | ==== | + | ====Materials==== |
# [http://parts.igem.org/Part:BBa_K1104205 ROS sensing system] | # [http://parts.igem.org/Part:BBa_K1104205 ROS sensing system] | ||
#T7 system | #T7 system | ||
| Line 48: | Line 50: | ||
[[image:NYMU_NO7.EXP.EXPERIMENT.png]] | [[image:NYMU_NO7.EXP.EXPERIMENT.png]] | ||
| - | ==== | + | ====Methods==== |
Before the experiment, we placed 1 ul bacteria liquid into 2 ml LB and put them into incubator for experiment on the next day. After growing the bacteria, we sucked a few liquid we cultured before, testing the OD value respectively. If the OD value is lower than 0.5, put the liquid into incubator again, repeating the step until the OD value is up to 0.5. In every well, we add mixture of M9 buffer and H2O2. The concentration of H2O2 is 1mM where is the best range where ROS promoter could switch on. Then we put control and experiment sample which have been cultured overnight and have the same OD value into wells. Then measure the OD and fluorescence value of samples through thermo varioskan flash. After using thermo varioskan flash , put 96 wells back to incubator for another 1 hour. Repeat the measuring and culturing steps for 10 points. At last, analyze the data with wavelength of green and red light. | Before the experiment, we placed 1 ul bacteria liquid into 2 ml LB and put them into incubator for experiment on the next day. After growing the bacteria, we sucked a few liquid we cultured before, testing the OD value respectively. If the OD value is lower than 0.5, put the liquid into incubator again, repeating the step until the OD value is up to 0.5. In every well, we add mixture of M9 buffer and H2O2. The concentration of H2O2 is 1mM where is the best range where ROS promoter could switch on. Then we put control and experiment sample which have been cultured overnight and have the same OD value into wells. Then measure the OD and fluorescence value of samples through thermo varioskan flash. After using thermo varioskan flash , put 96 wells back to incubator for another 1 hour. Repeat the measuring and culturing steps for 10 points. At last, analyze the data with wavelength of green and red light. | ||
====Result==== | ====Result==== | ||
*Testing result of positive feedback produced by T7 system([http://parts.igem.org/Part:BBa_K1104702 BBa_K1104702]) | *Testing result of positive feedback produced by T7 system([http://parts.igem.org/Part:BBa_K1104702 BBa_K1104702]) | ||
| + | |||
===Test the lethal dose of ethanol to honey bees=== | ===Test the lethal dose of ethanol to honey bees=== | ||
| Line 66: | Line 69: | ||
#Minimum inhibitory concentration for E.coli 2.73 uL / in 33uL body fluid[http://www.scielo.br/pdf/bjps/v45n2/v45n2a08.pdf] | #Minimum inhibitory concentration for E.coli 2.73 uL / in 33uL body fluid[http://www.scielo.br/pdf/bjps/v45n2/v45n2a08.pdf] | ||
| - | ==== | + | ====Materials==== |
#Honey bees | #Honey bees | ||
#Sucrose solution | #Sucrose solution | ||
#EtOH 95%, density=0.8 | #EtOH 95%, density=0.8 | ||
#Color paintings, red and green | #Color paintings, red and green | ||
| - | ==== | + | ====Methods==== |
#Paper survey, finding out EtOH lethal dose and sub-lethal dose for Bees | #Paper survey, finding out EtOH lethal dose and sub-lethal dose for Bees | ||
#Prepare EtOH sucrose solution: | #Prepare EtOH sucrose solution: | ||
| Line 91: | Line 94: | ||
|} | |} | ||
| - | ==== | + | ====Results==== |
2 uL ethanol is enough to kill the bees. Comparing to ''Bee. coli'', who need at least 2.73 uL ethanol to inhibit growth, honey bees are more fragile than ''Bee. coli''. | 2 uL ethanol is enough to kill the bees. Comparing to ''Bee. coli'', who need at least 2.73 uL ethanol to inhibit growth, honey bees are more fragile than ''Bee. coli''. | ||
| Line 101: | Line 104: | ||
===Progress of ethanol producing=== | ===Progress of ethanol producing=== | ||
So far, except promoter, we have pieced up most enzymes for producing ethanol. We planed to construct circuit below. | So far, except promoter, we have pieced up most enzymes for producing ethanol. We planed to construct circuit below. | ||
| - | + | [[File:nymu_no.7_酒精.png|800px|''' Circuit for testing alcohol-producing efficiency''']] <br> | |
| - | [[File: | + | We used HPLC to detect the alcohol with our equipment. Besides, we compared the reference which conducted by Jinchuan Yang before.[http://www.waters.com/webassets/cms/library/docs/720001896en.pdf] Here is Jinchuan Yang's way, result and relative parameter he used in detail. |
| + | *The protocol is set up by Waters corporation | ||
| + | *7.8X150mm IC-Pak, iron exclusion column (WAT010295) | ||
| + | *Mobile phase: 100% 0.1mM sulfuric acid | ||
| + | *Flow rate: 1.0mL/ min<br> | ||
| + | [[File:nymu_etoh5.png|800px|]] | ||
| + | Comparing to the reference, we conducted the same experiment with different sample and equipment. There is our parameter and result below. | ||
| + | *Preparative column,Delta-Pak C18 guard cartridge and column(25 mm i.d. × 10 mm length and 25 mm × 100 mm, respectively | ||
| + | *Mobile phase: 100% 0.1mM sulfuric acid | ||
| + | *Flow rate: 2.0mL/ min<br> | ||
| + | [[File:nymu_etoh6.png|800px|]]<br> | ||
| + | A solvent peak at 4.24 min is noticed. We concluded that the alcohol did not maintained by our column. In the future, some points we should improve are as below. | ||
| + | #Change to IC- column | ||
| + | #Establish LB medium standard | ||
| + | #Detect EtOH containing medium (culture medium with Bee. coli) | ||
| + | |||
| + | |||
| + | ===Progress of Cry toxin testing=== | ||
| + | We designed primer to clone the Cry toxin gene from ''Bacillus Thuringiensis Israelensis'', and the vector with constitutive promoter, reporter, ribosome binding site, and terminators from ''E. coli''. We have ligated both part with blind cutting sites.(Because of reporter on the vector, we could know whether they were legated reversely or not after checked with colony PCR which uses vector_FP and Cry_RP.) However, we faced some trouble in culturing the colony. DH5a or MG1655 is the target where we sent our engineered plasmid DNA. Both of them are gram-negative bacteria ,most of which cannot form endospores. As far as we know, when ''Bacillus Thuringiensis Israelensis'' produces Cry toxin, it will transform into endospore. And Cry toxin create unregulated pores in the membrane of targeted cells. We assumed the engineered plasmid did be put into our competent cells, but the Cry toxin derived from our engineered circuit kill the competent cells and other bacteria on the plates.(In fact, our competent cell have ever been contaminated by unknown plasmid carrying ampicillin or chloramphenicol resistance.) So, there is no colony growing on our plate. Our future plan is to book or make competent cells which are gram-positive bacteria, and proceeding our function assay on the midgut cells of honey bees. | ||
| + | [[File:Nymu_no.7_cry_toxin.png|800px|''' Circuit for testing lethality of Cry toxin ''']] | ||
| + | |||
==Reference== | ==Reference== | ||
#Mazzola, P. G., Jozala, A. F., Novaes, L. C. L., Moriel, P., & Penna, T. C. V. (June 01, 2009). Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Brazilian Journal of Pharmaceutical Sciences, 45, 2, 241-248.[http://www.jbc.org/content/66/1/77.full.pdf] | #Mazzola, P. G., Jozala, A. F., Novaes, L. C. L., Moriel, P., & Penna, T. C. V. (June 01, 2009). Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Brazilian Journal of Pharmaceutical Sciences, 45, 2, 241-248.[http://www.jbc.org/content/66/1/77.full.pdf] | ||
Latest revision as of 03:06, 29 October 2013


Contents |
Suicide Solution
Project overview
Introduction
Our aim is to resolve collapse colony disorder, which has been characterized in recent years. However, it is a quite up-to-date disease, researchers have found some suspicious candidates resulting CCD. Our project focuses on one of main pathogens, Nosema ceranae. As the previous group stated, we used theoretically plausible sensor to sense Nosema ceranae, molecules to prevent Nosema ceranae from invading, and even molecules to kill Nosema ceranae. In case of uselessness of previous circuit on Nosema ceranae, we have to come up with another way to hold back the spreading of Nosema ceranae. This is what we concentrate on.
Background
- Ethanol is known as a kind of toxins to bees. Up to certain degree, it has potential harm to the nervous system of bees. Besides, it could have influence on hydrostatical pressure, which is one of essential factors to the germination of Nosema ceranae.
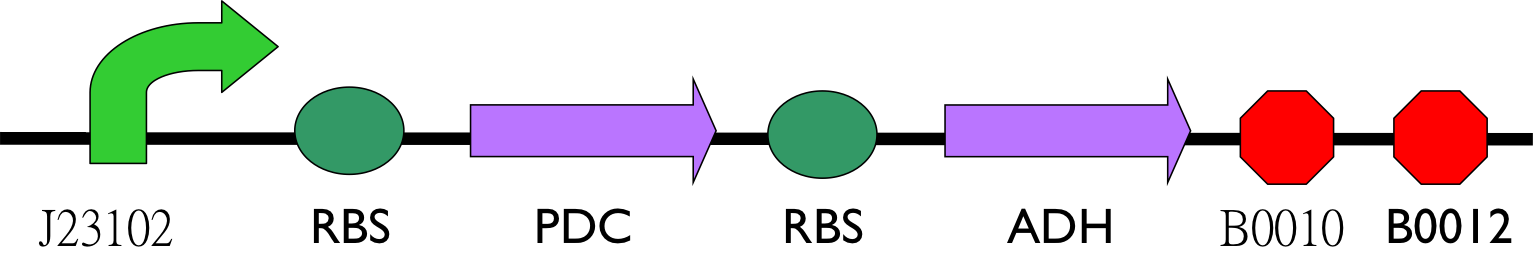
- Ethanol can be produced from pyruvate through [http://parts.igem.org/Part:BBa_K1104400 Pyruvate Decarboxylase (PDC)] and [http://parts.igem.org/Part:BBa_K1104401 Alcohol Dehydrogenase (ADH)].
- Cry toxins have specific activities against insect species of the orders Lepidoptera (moths and butterflies), Diptera (flies and mosquitoes), Coleoptera (beetles), Hymenoptera (wasps, bees, ants and sawflies) and nematodes.
- When insects ingest toxin crystals, the alkaline pH of their digestive tract denatures the insoluble crystals, making them soluble and thus amenable to being cut with proteases found in the insect gut, which liberate the cry toxin from the crystal. The Cry toxin is then inserted into the insect gut cell membrane, paralyzing the digestive tract and forming a pore. The insect stops eating and starves to death.
Methods and Circuit design
Methods
The infected honeybee can go back to their hive, contact other bees, and spread the pathogen in a very short time. To prevent the whole bees in hive from infected,we come up with a way to prohibit Nosema from speading. The method is composed of two parts.
- 1. How to post-sensing if Bee. coli could not successfully kill the Nosema.
We use the ROS promoter from the previous circuit. What difference between our circuit and previous circuit is we directly put two terminators after the ROS promoter to construct threshold. In case our circuit will be easily activated to cause unnecessary toll.
- 2. How to suppress the spreading of Nosema if Bee. coli failed
Inhibit the extrusion of the polar filament or let it germinate at the wrong place, and thus the spores cannot get into the intestine cell. Since the mechanism that triggers the polar filament’s extrusion is hydrostatic pressure, ethanol is our potential candidate for it is able to reduce water permeation across cell membrane and phospholipid layers, also caused a powerful inhibition of spore germination. (In this way, Nosema senses much lower hydrostatic pressure).
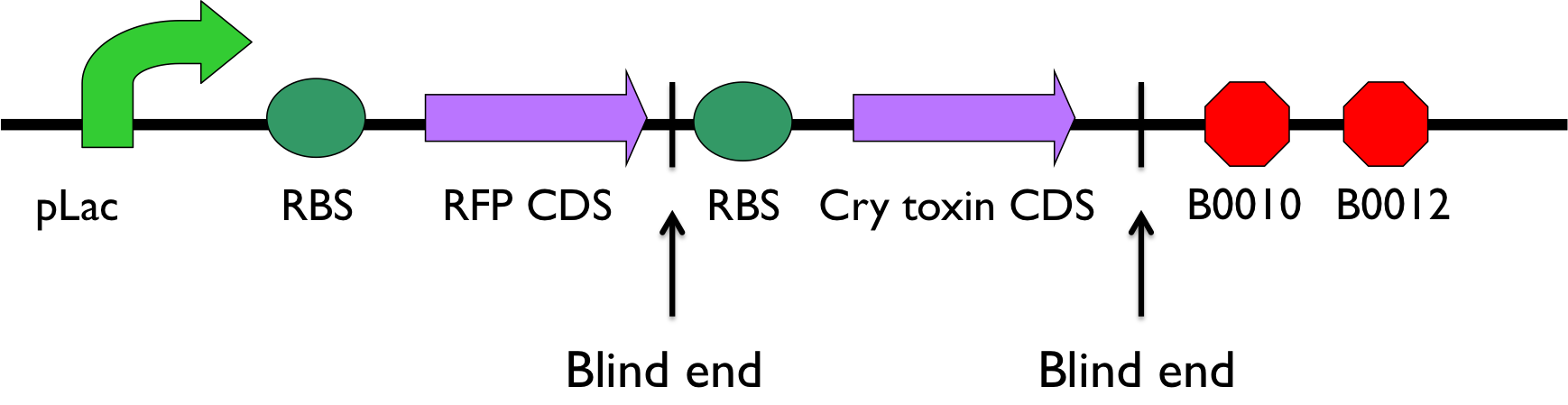
In addition, in order to prevent CCD from deteriorating, the another way is to sacrifice the infected bee for the good of the majority. That is, Bee. coli would secret lethal protein to kill the bee if the bee cannot be cured. We introduced Cry toxin as another candidate to kill the infected bee. According to assessment made by EPA(Environmental Protection Agency), the 5-day LC50 on honey bee require 7.0*107 cfu/g diet. However, the efficiency is not still good enough to stop the spreading of Nosema ceranae. We designed a positive feedback builded up by T7 promoter and T7 polymerase to shorten LC50 of honey bee. Besides, we cloned B0015 right after the ROS promoter in case bee accidentally killed by Bee. coli.
Circuit design
- According to the result of modeling, it is obvious that we should put 1~2 terminators whose forward efficiency is about 0.98 CC after our hybrid promoter. The purpose of the terminators is to prevent bees from dying easily.The purpose of the terminators is to prevent bees from dying easily. On top of that, the other important part is the positive feedback circuit. It is useless that we only produce very few pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) or Cry toxin. Hence we introduced some biobricks to our circuit to exert efficiency.
- The coding sequence of the first train is [http://parts.igem.org/Part:BBa_K145001 Part:BBa_K145001], which is the upstream DNA sequence of T7 polymerase. Otherwise, one of the coding sequence on the second strain will also be transcribed into the mRNA of T7 polymerase. In order to construct positive feedback of the alcohol-producing enzymes, we set a T7 polymerase coding sequence on the second train. Meanwhile in front of the second train, we place the receptor of T7 polymerase and [http://parts.igem.org/Part:BBa_I712074 T7 promoter]. In case of the leaky condition, we choose T7 polymerase as our go-between of alcohol-producing enzymes proliferating system.
Experiment
Test the producing efficiency with positive feedback
Introduction
- It is not enough to just produce enzymes to synthesize alcohol. We need a more abrupt, rapid way to produce PDC and ADH which were enzymes responsible for synthesizing alcohol, so we add T7 system on the original circuit to construct positive feedback.
Background
- According to the experiment made by 5th group, we could know the efficiency of ROS promoter
- According to the experiment made by Caitlin Conboy, we could know the forward efficiency of B0015[http://parts.igem.org/Help:Terminators/Measurement/Caitlin_Conboy]
Materials
- [http://parts.igem.org/Part:BBa_K1104205 ROS sensing system]
- T7 system
- [http://parts.igem.org/Part:BBa_B0015 B0015]
- [http://parts.igem.org/Part:BBa_E0840 GFP generator(E0840)]
- [http://parts.igem.org/Part:BBa_I13507 RFP generator(I13507)]
- H2O2
Outline
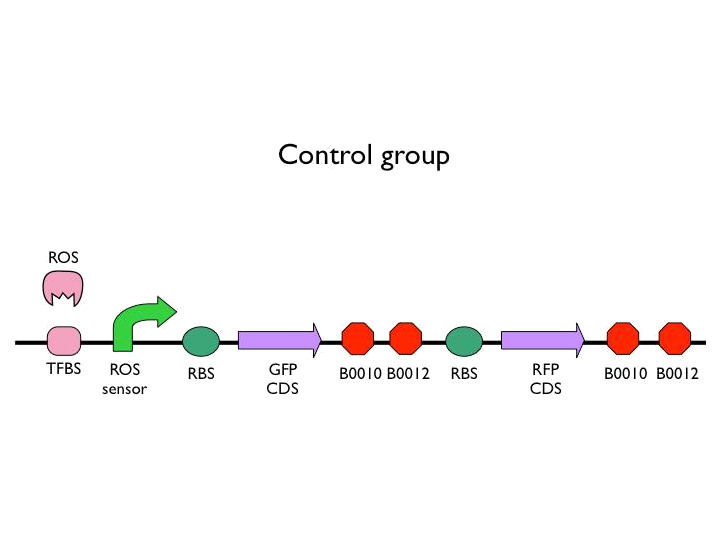
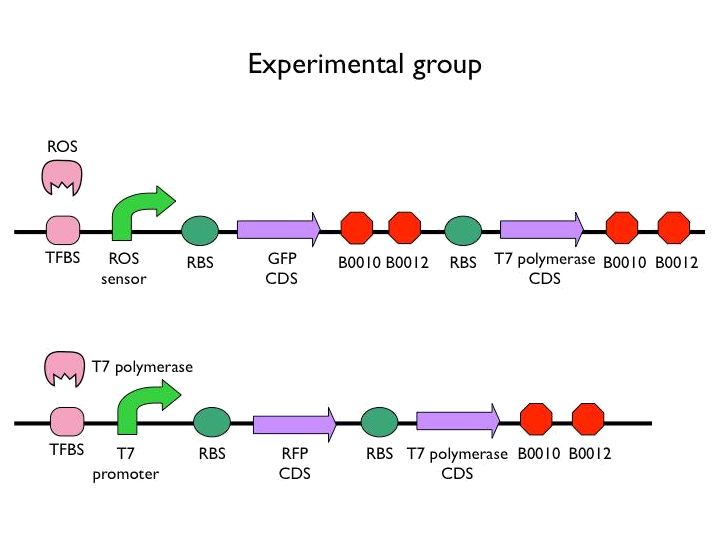
We created the circuit below. Every experiments are added the same concentration of H2O2. With reporter succeeding to the promoter, we could figure out whether the result was caused by promoter or not. And then there is the terminator we would test. After the terminator is the T7 polymerase which would open up the subsequent positive feedback. Besides, we used the other fluorescence protein as our second reporter. By testing the fluorescence from the second reporter, we could know how much proteins would be expressed on this site. Our control is the similar circuit without positive feedback below.
Methods
Before the experiment, we placed 1 ul bacteria liquid into 2 ml LB and put them into incubator for experiment on the next day. After growing the bacteria, we sucked a few liquid we cultured before, testing the OD value respectively. If the OD value is lower than 0.5, put the liquid into incubator again, repeating the step until the OD value is up to 0.5. In every well, we add mixture of M9 buffer and H2O2. The concentration of H2O2 is 1mM where is the best range where ROS promoter could switch on. Then we put control and experiment sample which have been cultured overnight and have the same OD value into wells. Then measure the OD and fluorescence value of samples through thermo varioskan flash. After using thermo varioskan flash , put 96 wells back to incubator for another 1 hour. Repeat the measuring and culturing steps for 10 points. At last, analyze the data with wavelength of green and red light.
Result
- Testing result of positive feedback produced by T7 system([http://parts.igem.org/Part:BBa_K1104702 BBa_K1104702])
Test the lethal dose of ethanol to honey bees
Introduction
In the midgut of the bees, where filled with ethanol, there is a big problem that whether Bee. coli could survive the ethanol before the bee died. To find the answer, we have to understand the persistence of the bee to ethanol.
Background
- Harnessed honey bees readily consume 1%, 5%, 10%, and 20% ethanol solutons.
- 95% ethanol will also be consumed as long as the antennae do not make contact with the solution.
- With the exception of 95% ethanol, consumption as measured by contact time or amount consumed does not differ in animals that consume 1%, 5%, 10%, and 20% ethanol solutions.
- Exposure to a lesser (or greater) concentration of ethanol does not influence consumption of a greater (or lesser) concentration.[http://www.alcoholism-cer.com/pt/re/alcoholism/abstract.00000374-200008000-00004.htm;jsessionid=SFFFMpK8gJJJJgpnHN4T2bGnS1NjQh1rx0pNc7mh0n5MfpJp7hBV!1424873047!181195629!8091!-1]
- Total body fluid inside one bee: 33 uL (ave.)[http://www.jbc.org/content/66/1/77.full.pdf]
- Minimum inhibitory concentration for E.coli 2.73 uL / in 33uL body fluid[http://www.scielo.br/pdf/bjps/v45n2/v45n2a08.pdf]
Materials
- Honey bees
- Sucrose solution
- EtOH 95%, density=0.8
- Color paintings, red and green
Methods
- Paper survey, finding out EtOH lethal dose and sub-lethal dose for Bees
- Prepare EtOH sucrose solution:
- Lethal dose, 40 uL 95% EtOH dissolved in 360 uL sucrose solution and feed 20 uL/bee
- Sub-lethal dose, 20L 95% EtOH dissolved in 380 uL sucrose solution and feed 20 uL/bee
- Normal control, 400 uL sucrose solution and feed 20 uL/bee
- Cooling bees by putting them into 4°C refrigerator
- Feeding bees with different conc. EtOH sucrose solution by 200uL tips
- Marker bees with different color painting, lethal dose: red, sub-lethal dose: green, normal control: non
- Put bees into bee hives and begin color run for 10 min
- Observation and record
| Bee's color run | Result of bee's color run |
|---|---|

| 
|
Results
2 uL ethanol is enough to kill the bees. Comparing to Bee. coli, who need at least 2.73 uL ethanol to inhibit growth, honey bees are more fragile than Bee. coli.
Future plan
Concerning possibility
According to the recent assay, it take 8 days from the invading of Nosema to the death of the bee. The time when suicide circuit activates should locate on around the 7th day, or the bee will be killed by Bee. coli. Based on modeling, our recent circuit design, which uses only two terminator with 0.98CC termination efficiency, it will activated the downstream positive feedback and kill the bee in 1 hour. We have concluded two ways to solve this problem.
- Put more terminators behind the ROS promoter. By this way, we could postpone the death of the bee. We will clone different amount of terminators and measure the efficiency of each group to decide the number of terminators we should clone.
- Select another promoter with weaker tendency to switch on. Currently, the T7 promoter we use has a quite high tendency. Therefore, the positive feedback will be switched on even very few ribosome going through the terminators.
Progress of ethanol producing
So far, except promoter, we have pieced up most enzymes for producing ethanol. We planed to construct circuit below.
We used HPLC to detect the alcohol with our equipment. Besides, we compared the reference which conducted by Jinchuan Yang before.[http://www.waters.com/webassets/cms/library/docs/720001896en.pdf] Here is Jinchuan Yang's way, result and relative parameter he used in detail.
- The protocol is set up by Waters corporation
- 7.8X150mm IC-Pak, iron exclusion column (WAT010295)
- Mobile phase: 100% 0.1mM sulfuric acid
- Flow rate: 1.0mL/ min
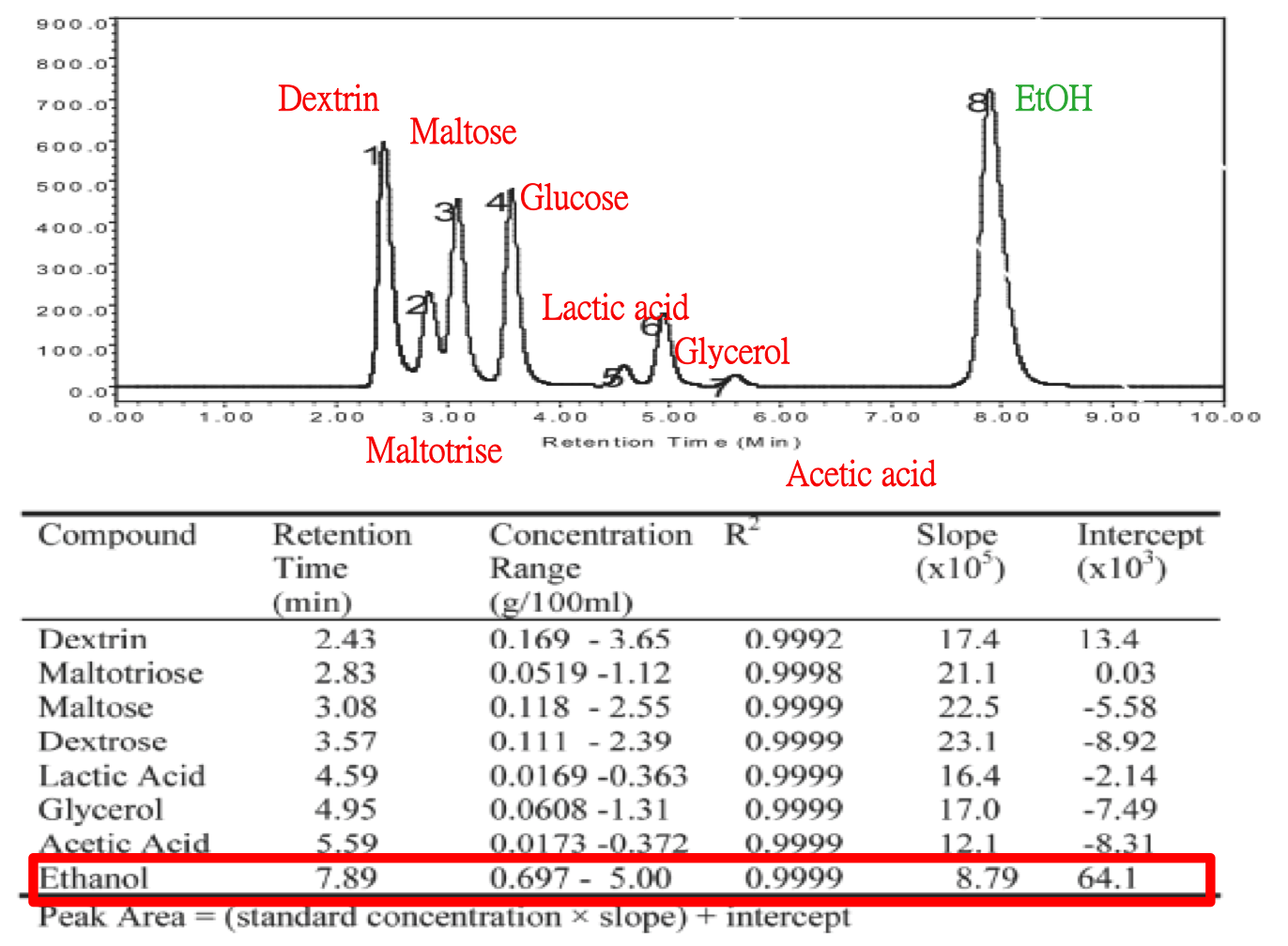 Comparing to the reference, we conducted the same experiment with different sample and equipment. There is our parameter and result below.
Comparing to the reference, we conducted the same experiment with different sample and equipment. There is our parameter and result below.
- Preparative column,Delta-Pak C18 guard cartridge and column(25 mm i.d. × 10 mm length and 25 mm × 100 mm, respectively
- Mobile phase: 100% 0.1mM sulfuric acid
- Flow rate: 2.0mL/ min

A solvent peak at 4.24 min is noticed. We concluded that the alcohol did not maintained by our column. In the future, some points we should improve are as below.
- Change to IC- column
- Establish LB medium standard
- Detect EtOH containing medium (culture medium with Bee. coli)
Progress of Cry toxin testing
We designed primer to clone the Cry toxin gene from Bacillus Thuringiensis Israelensis, and the vector with constitutive promoter, reporter, ribosome binding site, and terminators from E. coli. We have ligated both part with blind cutting sites.(Because of reporter on the vector, we could know whether they were legated reversely or not after checked with colony PCR which uses vector_FP and Cry_RP.) However, we faced some trouble in culturing the colony. DH5a or MG1655 is the target where we sent our engineered plasmid DNA. Both of them are gram-negative bacteria ,most of which cannot form endospores. As far as we know, when Bacillus Thuringiensis Israelensis produces Cry toxin, it will transform into endospore. And Cry toxin create unregulated pores in the membrane of targeted cells. We assumed the engineered plasmid did be put into our competent cells, but the Cry toxin derived from our engineered circuit kill the competent cells and other bacteria on the plates.(In fact, our competent cell have ever been contaminated by unknown plasmid carrying ampicillin or chloramphenicol resistance.) So, there is no colony growing on our plate. Our future plan is to book or make competent cells which are gram-positive bacteria, and proceeding our function assay on the midgut cells of honey bees.
Reference
- Mazzola, P. G., Jozala, A. F., Novaes, L. C. L., Moriel, P., & Penna, T. C. V. (June 01, 2009). Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Brazilian Journal of Pharmaceutical Sciences, 45, 2, 241-248.[http://www.jbc.org/content/66/1/77.full.pdf]
- Bishop, George H. (2011). Body fluid of the honey bee larva - II. Chemical constituents of the blood, and their osmotics effects.[http://www.scielo.br/pdf/bjps/v45n2/v45n2a08.pdf]
- Abramson, C. I., Stone, S. M., Ortez, R. A., Luccardi, A., Vann, K. L., Hanig, K. D., & Rice, J. (January 01, 2000). Neurobiological, Psychosocial, and Developmental Correlates of Drinking - The Development of an Ethanol Model Using Social Insects I: Behavior Studies of the Honey Bee (Apis mellifera L.). Alcoholism: Clinical and Experimental Research, 24, 8, 1153.[http://www.alcoholism-cer.com/pt/re/alcoholism/abstract.00000374-200008000-00004.htm;jsessionid=SDQRC8TrDPbvHJMqlm5qd7QQG3hvpyn7whPnqQ7LG2YPnQpDCQfs!1424873047!181195629!8091!-1]
- Higes, M., García-Palencia, P., Martín-Hernández, R., & Meana, A. (March 01, 2007). Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). Journal of Invertebrate Pathology, 94, 3, 211-217.[http://www.ncbi.nlm.nih.gov/pubmed/17217954]
- FRIXIONE, E. U. G. E. N. I. O., RUIZ, L. O. U. R. D. E. S., CERBÓN, J. O. R. G. E., & UNDEEN, A. L. B. E. R. T. H. (March 01, 1997). Germination of Nosema algerae (Microspora) Spores: Conditional Inhibition by D2O, Ethanol and Hg2+ Suggests Dependence of Water Influx upon Membrane Hydration and Specific Transmembrane Pathways. The Journal of Eukaryotic Microbiology, 44, 2, 109-116.[http://onlinelibrary.wiley.com/doi/10.1111/j.1550-7408.1997.tb05946.x/full]
- Registry of standard biological parts, BBa_K558001.[http://igem.ym.edu.tw/pv2013/index.php/Team:NYMU-Taipei/Nosema_spread_prevention#Method]
- 2010igem.UT-Tokyo, Sudoku_assay_LeakSw.[1]
- Registry of standard biological parts, Help:Terminators/Measurement/Caitlin Conboy[http://parts.igem.org/Help:Terminators/Measurement/Caitlin_Conboy]
- Höfte, H, & Whiteley, H R. (n.d.). Insecticidal crystal proteins of Bacillus thuringiensis.[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC372730/]
- United States. (1998). RED facts: Bacillus thuringensis. Washington, DC: U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances.[http://www.epa.gov/oppsrrd1/REDs/0247.pdf]
- Registry of standard biological parts, BBa_K332011.[http://parts.igem.org/Part:BBa_K332011:Experience]
- Dean, D. H. (1984). Biochemical genetics of the bacterial insect-control agent bacillus thuringiensis: Basic principles and prospects for genetic engineering. Newcastle upon Tyne: Intercept Ltd.[http://www.nottingham.ac.uk/ncmh/BGER/pdf/Volume%202/BGER2-12.pdf]
- J. Yang,Fast hplc analysis for fermentation ethanol processes - Waters[http://www.waters.com/webassets/cms/library/docs/720001896en.pdf]
 "
"