Team:British Columbia/Project/Cinnamaldehyde
From 2013.igem.org
AnnaMüller (Talk | contribs) (→What purpose Does it Have in our Project) |
Joelkumlin (Talk | contribs) (→Cinnamaldehyde) |
||
| (33 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:British_Columbia/Templates/MainHeader}} | {{:Team:British_Columbia/Templates/MainHeader}} | ||
| + | <html> | ||
| + | <a name="top"></a> | ||
| + | </html> | ||
=Cinnamaldehyde= | =Cinnamaldehyde= | ||
| - | Cinnamaldehyde makes up about 65% of cinnamon oil, which is extracted from the bark of cinnamon | + | Cinnamaldehyde makes up about 65% of cinnamon oil, which is extracted from the bark of cinnamon trees, and it provides the characteristic flavor and aroma to cinnamon products (1). The potential uses of cinnamaldehyde go far beyond its common flavor and aroma properties a review of the literature will show that cinnamaldehyde has characteristics that make it a potential food safety additive as well as a type two diabetes therapeutic. |
| - | Though cinnamon has been used to keep food from spoiling in parts of India and China for years, scientific evidence has only recently been able support these claims. To date cinnamaldehyde has been shown to inhibit growth of common food born pathogens such as; <i>Lysteria monocytogenes</i>, various <i>Salmonella</i> species, <i>E. Coli</i> O157:H7, as well as viruses, and some fungi(2,3) Yet, members of the <i>Lactobacillus</i> family, such as lactobacillus sakei, have demonstrated resistance to cinnamaldehyde up to 0. | + | Though cinnamon has been used to keep food from spoiling in parts of India and China for years, scientific evidence has only recently been able support these claims. To date cinnamaldehyde has been shown to inhibit growth of common food born pathogens such as; <i>Lysteria monocytogenes</i>, various <i>Salmonella</i> species, <i>E. Coli</i> O157:H7, as well as viruses, and some fungi (2,3) Yet, members of the <i>Lactobacillus</i> family, such as lactobacillus sakei, have demonstrated resistance to cinnamaldehyde up to 0.5 M, much lower than the 30 mM needed to inhibit listeria, suggesting that its antimicrobial properties could be hosted in our lactobacillus bulgaris chasis (3). Thus, one could imagine a biosynthetic approach to creating cinnamaldehyde would provide the food industry with a useful tool to make food safer. |
| - | This antimicrobial activity has applications to human health as well. <i>Heliobacter pylori</i> is a common pathogen found in the stomach that is responsible for pH induced gastritis and peptic ulcers. It is estimated that ½ the world’s population is infected with <i>Heliobacter pylori</i> (3). In severe cases, such as those often found in the third world, <i>Heliobacter pylori</i> can result in gastric cancer formation. <i>In vitro</i> studies however, have shown that doses of cinnamaldehyde at | + | This antimicrobial activity has applications to human health as well. <i>Heliobacter pylori</i> is a common pathogen found in the stomach that is responsible for pH induced gastritis and peptic ulcers. It is estimated that ½ the world’s population is infected with <i>Heliobacter pylori</i> (3). In severe cases, such as those often found in the third world, <i>Heliobacter pylori</i> can result in gastric cancer formation. <i>In vitro</i> studies however, have shown that doses of cinnamaldehyde at 2 ug/ml are capable of wiping out said bacterium. Furthermore, evidence suggests that bacterial resistance to cinnamaldehyde does not develop easily (3). This suggests that cinnamaldehyde might be a potent alternative to antibiotic therapy. Though treating gastric illness is a potent application of this chemical entity, cinnamaldehyde offers a more impressive solution to an ongoing global epidemic: type II diabetes. |
| - | The World Health Organization (WHO) estimates that there are 347 million people globally living with diabetes, 90% of which suffer from type 2, as of 2030 diabetes is projected to be the 7th leading cause of death globally | + | The World Health Organization (WHO) estimates that there are 347 million people globally living with diabetes, 90% of which suffer from type 2, as of 2030 diabetes is projected to be the 7th leading cause of death globally (4). |
| - | It appears as though there is justification for the search for new and novel solutions to this ever-growing problem. In a study from 2007 mice suffering from type 2 diabetes showed a significant reduction in blood glucose, total cholesterol, | + | It appears as though there is justification for the search for new and novel solutions to this ever-growing problem. In a study from 2007 mice suffering from type 2 diabetes showed a significant reduction in blood glucose, total cholesterol, triglyceride levels, and an increase in plasma insulin levels when administered cinnamaldehyde orally (20 mg/kg)(5). Furthermore, it has been suggested that a 120 mg/day - 6 g/day dose of cinnamon can produce similar effects in humans (6). |
==Rationale== | ==Rationale== | ||
| Line 17: | Line 20: | ||
==Chemistry== | ==Chemistry== | ||
| - | <center> | + | <html><center><IMG id="emp" SRC="https://static.igem.org/mediawiki/2013/a/ad/UBC-Cinnamic-Acid-structure.png" USEMAP="#cinnamaldehyde"> |
| - | + | <map name="cinnamaldehyde" id="cinnamaldehyde"> | |
| - | </center> | + | <area shape="rect" coords="127,33,267,109" href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1129026" target="" /> |
| + | <area shape="rect" coords="337,56,474,108" href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1129042" target="" /> | ||
| + | <area shape="rect" coords="560,55,695,106" href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1129039" target="" /> | ||
| + | </map> | ||
| + | </center></html> | ||
==Characterization== | ==Characterization== | ||
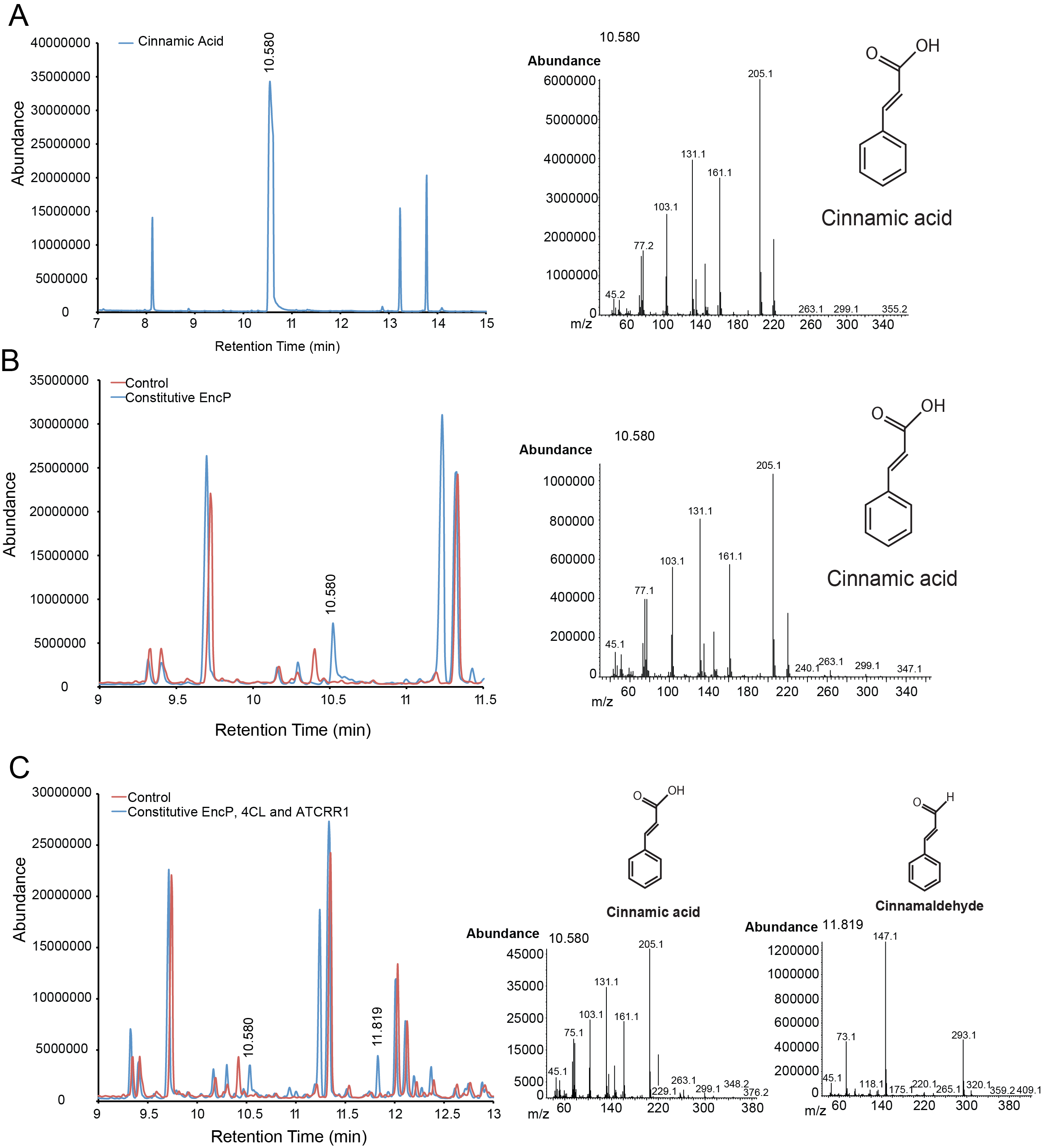
| + | For the parts in the cinnamaldehyde biosynthetic pathway, we characterized compound generation by CG-MS. We did whole cell transformation and gained evidence for in vivo transformations for our constitutively expressed EncP, 4CH and ATCRR1 which completes the pathway. As seen below, cinnamic acid was identified with an internal standard and mass spectrum comparisons. For cinnamaldehyde generation, the compound was identified by mass spectrum database matching with a probability of 46%. We are in the process of confirming cinnamaldehyde with an internal standard while running the necessary controls to being quantification of these compounds. As for our inducible constructs, we were not able to see conversion with 10 µg/mL of arabinose (see part characterization pages). We are titrating other levels of induction to see if the lack of compound generation is due to low expression. | ||
| - | [[File: | + | [[File:UBC_Cinnamaldehdye_wiki.png|900px]] |
| - | + | '''Figure 1.''' Compound generation identification by GC-MS. Chromatograms and mass spectra for select peaks are shown. Structures represent predictions based on library matching or comparison to standards. Controls represent plasmids missing the gene of interest. '''A.''' Internal control using cinnamic acid. '''B.''' cinamic acid detection from culture containing phenylalanine and strains harbouring constitutive EncP. '''C.''' Cinamic acid and cinnamaldehyde detection from culture containing phenylalanine and strains harboring constitutive EncP, 4CH and ATRCC1. | |
| - | |||
| - | + | <html><center> | |
| + | <img src="https://static.igem.org/mediawiki/2012/a/a3/UBCOthersds.png" width="400"> </img> </center> | ||
| - | |||
| - | |||
| - | 5) http://www.sciencedirect.com/science/article/pii/S0944711306001772) | + | <p><b>Figure 2.</b> 12% SDS-PAGE of protein samples from some of the cinnamaldehyde and vanillin enzymes being expressed under the pBad promoter. No expression was detected, however, due to the detection of products from GC-MS, it is likely that the enzymes are being expressed, but not at a high enough level to detect on the gel.</p> |
| + | |||
| + | </html> | ||
| + | |||
| + | |||
| + | <br> | ||
| + | |||
| + | <html> | ||
| + | |||
| + | <div> | ||
| + | <center> | ||
| + | |||
| + | <a href="https://2013.igem.org/Team:British_Columbia/Project/Flavours"> | ||
| + | <img width="180" class="icon" src="https://static.igem.org/mediawiki/2013/7/79/UBCReturnArrow.png" | ||
| + | onmouseover="this.src='https://static.igem.org/mediawiki/2013/d/d3/UBCReturnFlavour.png'" | ||
| + | onmouseout="this.src='https://static.igem.org/mediawiki/2013/7/79/UBCReturnArrow.png'"/></a> | ||
| + | </center> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | </html> | ||
| + | |||
| + | <br> | ||
| + | |||
| + | ==References== | ||
| + | |||
| + | <html> | ||
| + | 1) <a href="http://www.sciencedirect.com/science/article/pii/S0168160504001680">Burt, S. Essential oils: their antibacterial properties and potential applications in foods--a review. <i>Int. J. Food. Microbiol.</i> <b>94</b>, 223-253 (2004). </a> | ||
| + | <br> | ||
| + | <br> | ||
| + | 2) <a href="http://www.ann-clinmicrob.com/content/4/1/20">Ali, S.M., Khan, A.A., Ahmed, I., Musaddiq, M., Ahmed, K.S., Polasa, H., Rao, L.V., Habibullah, C.M., Sechi, L.A., & Ahmed, N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen <i>Helicobacter pylori</i>. <i>Ann. Clin. Microbiol. Antimicrob.</i> <b>4</b>, 20 (2005). </a> | ||
| + | <br> | ||
| + | <br> | ||
| + | 3) <a href="http://aem.asm.org/content/70/10/5750.full">Gill, A.O., & Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against <i>Listeria monocytogenes</i> and of eugenol against <i>L. monocytogenes</i> and <i>Lactobacillus sakei</i>. <i>Appl. Environ. Microbiol.</i> <b>70</b>, 5750-5755 (2004). </a> | ||
| + | <br> | ||
| + | <br> | ||
| + | 4) <a href="http://www.who.int/mediacentre/factsheets/fs312/en/">World Health Organization. (Retrieved August 2013). </a> | ||
| + | <br> | ||
| + | <br> | ||
| + | 5) <a href="http://www.sciencedirect.com/science/article/pii/S0944711306001772">Subash, B.P., Prabuseenivasan, S., & Ignacimuthu, S. Cinnamaldehyde--a potential antidiabetic agent. <i>Phytomedicine.</i> <b>14</b>, 15-22 (2006). </a> | ||
| + | <br> | ||
| + | <br> | ||
| + | 6) <a href="http://www.annfammed.org/content/11/5/452.long">Allen, R.W., Schwartzman, E., Baker, W.L., Coleman, C.I., & Phung, O.J. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. <i>Ann. Fam. Med.</i> <b>11</b>, 452-459 (2013). </a> | ||
| - | + | <br> | |
| + | <div align="right"><a href="#top">Top of Page</a></div> | ||
| + | </html> | ||
Latest revision as of 03:02, 29 October 2013
iGEM Home


Contents |
Cinnamaldehyde
Cinnamaldehyde makes up about 65% of cinnamon oil, which is extracted from the bark of cinnamon trees, and it provides the characteristic flavor and aroma to cinnamon products (1). The potential uses of cinnamaldehyde go far beyond its common flavor and aroma properties a review of the literature will show that cinnamaldehyde has characteristics that make it a potential food safety additive as well as a type two diabetes therapeutic.
Though cinnamon has been used to keep food from spoiling in parts of India and China for years, scientific evidence has only recently been able support these claims. To date cinnamaldehyde has been shown to inhibit growth of common food born pathogens such as; Lysteria monocytogenes, various Salmonella species, E. Coli O157:H7, as well as viruses, and some fungi (2,3) Yet, members of the Lactobacillus family, such as lactobacillus sakei, have demonstrated resistance to cinnamaldehyde up to 0.5 M, much lower than the 30 mM needed to inhibit listeria, suggesting that its antimicrobial properties could be hosted in our lactobacillus bulgaris chasis (3). Thus, one could imagine a biosynthetic approach to creating cinnamaldehyde would provide the food industry with a useful tool to make food safer.
This antimicrobial activity has applications to human health as well. Heliobacter pylori is a common pathogen found in the stomach that is responsible for pH induced gastritis and peptic ulcers. It is estimated that ½ the world’s population is infected with Heliobacter pylori (3). In severe cases, such as those often found in the third world, Heliobacter pylori can result in gastric cancer formation. In vitro studies however, have shown that doses of cinnamaldehyde at 2 ug/ml are capable of wiping out said bacterium. Furthermore, evidence suggests that bacterial resistance to cinnamaldehyde does not develop easily (3). This suggests that cinnamaldehyde might be a potent alternative to antibiotic therapy. Though treating gastric illness is a potent application of this chemical entity, cinnamaldehyde offers a more impressive solution to an ongoing global epidemic: type II diabetes.
The World Health Organization (WHO) estimates that there are 347 million people globally living with diabetes, 90% of which suffer from type 2, as of 2030 diabetes is projected to be the 7th leading cause of death globally (4).
It appears as though there is justification for the search for new and novel solutions to this ever-growing problem. In a study from 2007 mice suffering from type 2 diabetes showed a significant reduction in blood glucose, total cholesterol, triglyceride levels, and an increase in plasma insulin levels when administered cinnamaldehyde orally (20 mg/kg)(5). Furthermore, it has been suggested that a 120 mg/day - 6 g/day dose of cinnamon can produce similar effects in humans (6).
Rationale
The ubiquitous use of cinnamon in food products and aroma products, in combination with its potential benefit to food safety and our personal health through its make this compound an exciting one to target for synthesis and incorporation in our ultrabiotic. When combined with the other two flavoring compounds it offers us an opportunity to exhibit CRISPR’s ability to tune bacterial populations through immunity to phage. This mechanism of tunable populations is demonstrated in the “population control” section of our Wiki.
Chemistry

Characterization
For the parts in the cinnamaldehyde biosynthetic pathway, we characterized compound generation by CG-MS. We did whole cell transformation and gained evidence for in vivo transformations for our constitutively expressed EncP, 4CH and ATCRR1 which completes the pathway. As seen below, cinnamic acid was identified with an internal standard and mass spectrum comparisons. For cinnamaldehyde generation, the compound was identified by mass spectrum database matching with a probability of 46%. We are in the process of confirming cinnamaldehyde with an internal standard while running the necessary controls to being quantification of these compounds. As for our inducible constructs, we were not able to see conversion with 10 µg/mL of arabinose (see part characterization pages). We are titrating other levels of induction to see if the lack of compound generation is due to low expression.
Figure 1. Compound generation identification by GC-MS. Chromatograms and mass spectra for select peaks are shown. Structures represent predictions based on library matching or comparison to standards. Controls represent plasmids missing the gene of interest. A. Internal control using cinnamic acid. B. cinamic acid detection from culture containing phenylalanine and strains harbouring constitutive EncP. C. Cinamic acid and cinnamaldehyde detection from culture containing phenylalanine and strains harboring constitutive EncP, 4CH and ATRCC1.

Figure 2. 12% SDS-PAGE of protein samples from some of the cinnamaldehyde and vanillin enzymes being expressed under the pBad promoter. No expression was detected, however, due to the detection of products from GC-MS, it is likely that the enzymes are being expressed, but not at a high enough level to detect on the gel.
References
1) Burt, S. Essential oils: their antibacterial properties and potential applications in foods--a review. Int. J. Food. Microbiol. 94, 223-253 (2004).
2) Ali, S.M., Khan, A.A., Ahmed, I., Musaddiq, M., Ahmed, K.S., Polasa, H., Rao, L.V., Habibullah, C.M., Sechi, L.A., & Ahmed, N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 4, 20 (2005).
3) Gill, A.O., & Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 70, 5750-5755 (2004).
4) World Health Organization. (Retrieved August 2013).
5) Subash, B.P., Prabuseenivasan, S., & Ignacimuthu, S. Cinnamaldehyde--a potential antidiabetic agent. Phytomedicine. 14, 15-22 (2006).
6) Allen, R.W., Schwartzman, E., Baker, W.L., Coleman, C.I., & Phung, O.J. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann. Fam. Med. 11, 452-459 (2013).
 "
"