Team:Paris Saclay/Notebook/August/30
From 2013.igem.org
(→1 - Electrophoresis of PCR Colony of FNR, RBS-FNR and RBS-BphR2) |
|||
| (49 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
==='''A - Aerobic/Anaerobic regulation system'''=== | ==='''A - Aerobic/Anaerobic regulation system'''=== | ||
| - | ===='''Objective : characterize | + | ===='''Objective : characterize BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006'''==== |
| - | ===='''1 - Results of liquid culture of | + | ===='''1 - Results of liquid culture of Pndh*-RBS-Amil CP-Term in pSB1C3 in aerobic and anaerobic conditions'''==== |
XiaoJing | XiaoJing | ||
| Line 20: | Line 20: | ||
[[File:PsPfnr3008.jpg|600px]] | [[File:PsPfnr3008.jpg|600px]] | ||
| - | In anaerobic | + | In anaerobic condition, FNR protein (active form) binds to the constitutive promoter Pndh* and repressed ''amilCP'' expression. Therefore, the bacteria have no colour. |
| - | In aerobic | + | In aerobic condition, FNR protein is inactive and can not bind to constitutive promoter Pndh*, ''amilCP'' is expressed and we can see the bacteria have violet colour. |
| - | ===='''2 - Results of culture of | + | ===='''2 - Results of culture of Pndh* with RBS-AmilCP-Term in pSB1C3, PnirB with RBS-LacZ-Term in pSB1C3 in aerobic and anaerobic conditions'''==== |
XiaoJing | XiaoJing | ||
| Line 30: | Line 30: | ||
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DE;" | | | style="border:1px solid black;padding:5px;background-color:#DE;" | | ||
| - | Purification of 08/ | + | Purification of 08/29/13 didn't work. We have blue colonies for Pndh* with RBS-AmilCP-Term in pSB1C3 in aerobic and anaerobic conditions. We also have blue colonies for PnirB with RBS-LacZ-Term in pSB1C3 in anaerobic conditions. |
|} | |} | ||
| Line 36: | Line 36: | ||
[[File:PsNirBcult3008.jpg|500px]] | [[File:PsNirBcult3008.jpg|500px]] | ||
| - | ===='''3 - Ligation of | + | ===='''3 - Ligation of Pndh* with RBS-LacZ-Term in pSB1C3 '''==== |
XiaoJing | XiaoJing | ||
Used quantities : | Used quantities : | ||
| - | * | + | * Pndh* : 8µL |
| - | * RBS-LacZ-Term in | + | * RBS-LacZ-Term in pSB1C3 : 3µL |
* Buffer ligation : 2µL | * Buffer ligation : 2µL | ||
* Ligase : 1µL | * Ligase : 1µL | ||
* H2O : 6µL | * H2O : 6µL | ||
| - | ===='''4 - Transformation of ligation of | + | ===='''4 - Transformation of ligation of Pndh* with RBS-LacZ-Term in pSB1C3 in DH5α strain '''==== |
XiaoJing | XiaoJing | ||
| Line 53: | Line 53: | ||
Protocol : [[Team:Paris_Saclay/Protocols/Transformation|Bacterial transformation]] | Protocol : [[Team:Paris_Saclay/Protocols/Transformation|Bacterial transformation]] | ||
| - | We incubate | + | We incubate the bacteria in LB-chloramphenicol-Xgal plate at 37°C in aerobic conditions. |
| - | + | ===='''5 - Ligation of Pfnr with RBS-LacZ-Term in pSB3K3 and PnarK with RBS-LacZ-Term in pSB3K3 '''==== | |
| - | + | ||
| - | + | ||
XiaoJing | XiaoJing | ||
Used quantities : | Used quantities : | ||
| - | * | + | * Pndh*, PnarK : 3µL |
* RBS-LacZ-Term : 3µL | * RBS-LacZ-Term : 3µL | ||
| - | * | + | * pSB3K3 : 5µL |
* Buffer ligase : 2µL | * Buffer ligase : 2µL | ||
* Ligase : 1µL | * Ligase : 1µL | ||
| - | * H2O : 6µL | + | * H2O : 6µL |
| - | ====''' | + | ===='''6 - Transformation of ligation of Pndh* with RBS-LacZ-Term in pSB3K3 and PnarK with RBS-LacZ-Term in pSB3K3 in DH5α strain '''==== |
XiaoJing | XiaoJing | ||
| Line 75: | Line 73: | ||
Protocol : [[Team:Paris_Saclay/Protocols/Transformation|Bacterial transformation]] | Protocol : [[Team:Paris_Saclay/Protocols/Transformation|Bacterial transformation]] | ||
| - | We incubate | + | We incubate the bacteria at 37°C with LB-Kanamycine-Xgal in anaerobic conditions for PnarK with RBS-LacZ-Term in pSB3K3. |
| + | |||
| + | We incubate the bacteria at 37°C with LB-Kanamycine-Xgal in aerobic conditions for Pndh* with RBS-LacZ-Term in pSB3K3. | ||
| + | |||
| + | ===='''7 - Electrophoresis of PCR Colony of Pndh* with RBS_AmilCP-Term in DH5α strain '''==== | ||
| + | |||
| + | XiaoJing | ||
| + | |||
| + | {| | ||
| + | | style="width:350px;border:1px solid black;" | [[File:Psgel13008.jpg]] | ||
| + | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
| + | * Well 1 : 6µL DNA Ladder | ||
| + | * Well 2 : 20µL of Pndh* with RBS_AmilCP-Term clone 1 + 4µL of 6X loading dye | ||
| + | * Well 3 : 20µL of Pndh* with RBS_AmilCP-Term clone 2 + 4µL of 6X loading dye | ||
| + | * Well 4 : 20µL of Pndh* with RBS_AmilCP-Term clone 3 + 4µL of 6X loading dye | ||
| + | * Well 5 : 20µL of Pndh* with RBS_AmilCP-Term clone 4 + 4µL of 6X loading dye | ||
| + | * Gel : 1.0% | ||
| + | |} | ||
| + | |||
| + | Expected sizes : | ||
| + | * Pndh* with RBS_AmilCP-Term : 1000bp | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| + | We obtained fragments at the right size. | ||
| + | |} | ||
| + | |||
| + | ===='''8 - Result of the purification colony of MG1655Z1 Δfnr'''==== | ||
| + | |||
| + | XiaoJing | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DE;" | | ||
| + | It works. Our plasmid didn't contain any antibiotic resistance gene. Colonies grew in LB medium and didn't grow on kanamycin, ampicilin, chloramphenicol medium. We obtain strain MG1655Z1 Δfnr. | ||
| + | |} | ||
| + | |||
| + | [[File:PsLB3008.jpg|311px]][[File:PsKm3008.jpg|300px]] | ||
| + | [[File:PsCm3008.jpg|311px]][[File:PsAm3008.jpg|311px]] | ||
==='''A - Aerobic/Anaerobic regulation system / B - PCB sensor system'''=== | ==='''A - Aerobic/Anaerobic regulation system / B - PCB sensor system'''=== | ||
| Line 81: | Line 116: | ||
===='''Objective : obtaining FNR and BphR2 proteins'''==== | ===='''Objective : obtaining FNR and BphR2 proteins'''==== | ||
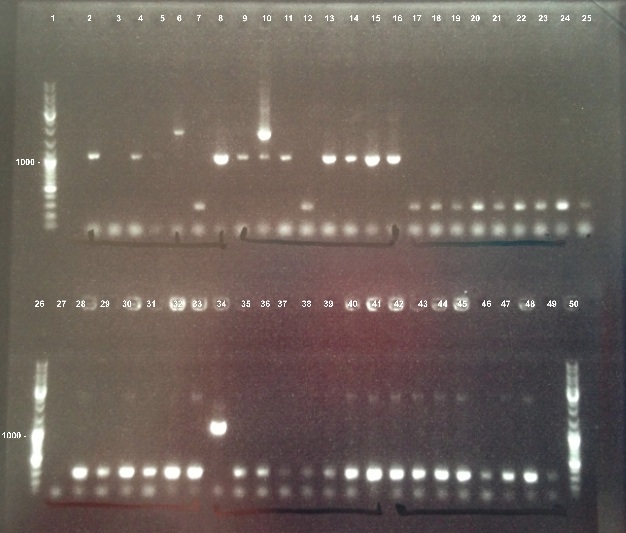
| - | ===='''1 - Electrophoresis of | + | ===='''1 - Electrophoresis of PCR Colony of FNR, RBS-FNR and RBS-BphR2'''==== |
{| | {| | ||
| - | | style="width:350px;border:1px solid black;" |]] | + | | style="width:350px;border:1px solid black;" |[[File:Psgel23008.jpg]] |
| style="width:350px;border:1px solid black;vertical-align:top;" | | | style="width:350px;border:1px solid black;vertical-align:top;" | | ||
* Well 1 : 6µL DNA Ladder | * Well 1 : 6µL DNA Ladder | ||
| - | * Well 2 to 17 : 5µL of FNR+1µl of 6X loading dye | + | * Well 2 to 17 : 5µL of FNR + 1µl of 6X loading dye |
| - | * Well 18 to 26 : 5µL of RBS-BphR2+1µl of 6X loading dye | + | * Well 18 to 26 : 5µL of RBS-BphR2 + 1µl of 6X loading dye |
* Well 27 : 6µL DNA Ladder | * Well 27 : 6µL DNA Ladder | ||
| - | * Well 28 to 41 : 5µL of RBS-FNR+1µl of 6X loading dye | + | * Well 28 to 41 : 5µL of RBS-FNR + 1µl of 6X loading dye |
| - | * Well 42 to 49 : 5µL of RBS-BphR2+1µl of 6X loading dye | + | * Well 42 to 49 : 5µL of RBS-BphR2 + 1µl of 6X loading dye |
| - | * | + | * Well 50 : 6µL DNA Ladder |
* Gel : 1% | * Gel : 1% | ||
|} | |} | ||
| Line 103: | Line 138: | ||
{| | {| | ||
| style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | | style="border:1px solid black;padding:5px;background-color:#DEDEDE;" | | ||
| - | We | + | We obtained fragments at the right size for FNR and RBS-FNR. The Gisbon assembly of 08/26/13 was good. |
|} | |} | ||
| Line 112: | Line 147: | ||
|[[Team:Paris_Saclay/Notebook|<big>Back to calendar</big>]] | |[[Team:Paris_Saclay/Notebook|<big>Back to calendar</big>]] | ||
| - | |[[Team:Paris Saclay/Notebook/ | + | |[[Team:Paris Saclay/Notebook/August/31|<big>Next day</big>]] |
|} | |} | ||
{{Team:Paris_Saclay/incl_fin}} | {{Team:Paris_Saclay/incl_fin}} | ||
Latest revision as of 01:23, 5 October 2013
Notebook : August 30
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize BBa_K1155000, BBa_K1155004, BBa_K1155005, BBa_K1155006
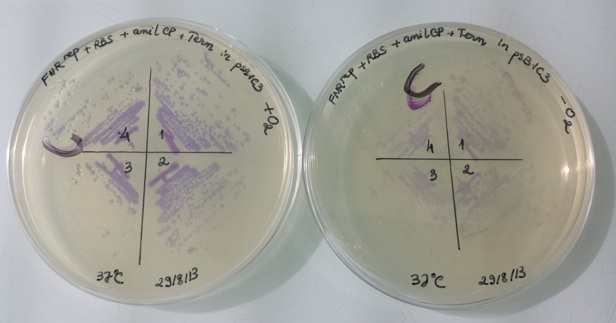
1 - Results of liquid culture of Pndh*-RBS-Amil CP-Term in pSB1C3 in aerobic and anaerobic conditions
XiaoJing
|
IT WORKS !!! |
In anaerobic condition, FNR protein (active form) binds to the constitutive promoter Pndh* and repressed amilCP expression. Therefore, the bacteria have no colour.
In aerobic condition, FNR protein is inactive and can not bind to constitutive promoter Pndh*, amilCP is expressed and we can see the bacteria have violet colour.
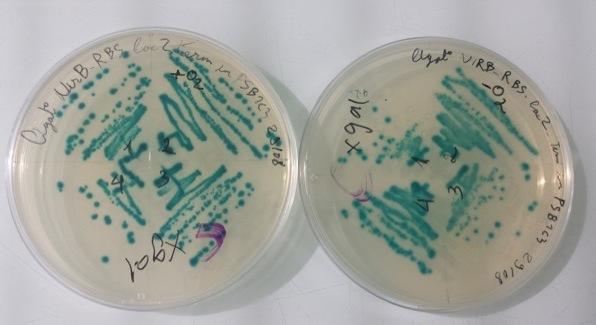
2 - Results of culture of Pndh* with RBS-AmilCP-Term in pSB1C3, PnirB with RBS-LacZ-Term in pSB1C3 in aerobic and anaerobic conditions
XiaoJing
|
Purification of 08/29/13 didn't work. We have blue colonies for Pndh* with RBS-AmilCP-Term in pSB1C3 in aerobic and anaerobic conditions. We also have blue colonies for PnirB with RBS-LacZ-Term in pSB1C3 in anaerobic conditions. |
3 - Ligation of Pndh* with RBS-LacZ-Term in pSB1C3
XiaoJing
Used quantities :
- Pndh* : 8µL
- RBS-LacZ-Term in pSB1C3 : 3µL
- Buffer ligation : 2µL
- Ligase : 1µL
- H2O : 6µL
4 - Transformation of ligation of Pndh* with RBS-LacZ-Term in pSB1C3 in DH5α strain
XiaoJing
Protocol : Bacterial transformation
We incubate the bacteria in LB-chloramphenicol-Xgal plate at 37°C in aerobic conditions.
5 - Ligation of Pfnr with RBS-LacZ-Term in pSB3K3 and PnarK with RBS-LacZ-Term in pSB3K3
XiaoJing
Used quantities :
- Pndh*, PnarK : 3µL
- RBS-LacZ-Term : 3µL
- pSB3K3 : 5µL
- Buffer ligase : 2µL
- Ligase : 1µL
- H2O : 6µL
6 - Transformation of ligation of Pndh* with RBS-LacZ-Term in pSB3K3 and PnarK with RBS-LacZ-Term in pSB3K3 in DH5α strain
XiaoJing
Protocol : Bacterial transformation
We incubate the bacteria at 37°C with LB-Kanamycine-Xgal in anaerobic conditions for PnarK with RBS-LacZ-Term in pSB3K3.
We incubate the bacteria at 37°C with LB-Kanamycine-Xgal in aerobic conditions for Pndh* with RBS-LacZ-Term in pSB3K3.
7 - Electrophoresis of PCR Colony of Pndh* with RBS_AmilCP-Term in DH5α strain
XiaoJing
Expected sizes :
- Pndh* with RBS_AmilCP-Term : 1000bp
|
We obtained fragments at the right size. |
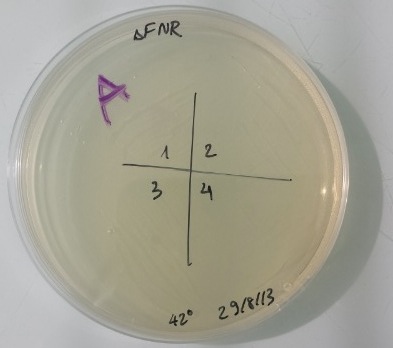
8 - Result of the purification colony of MG1655Z1 Δfnr
XiaoJing
|
It works. Our plasmid didn't contain any antibiotic resistance gene. Colonies grew in LB medium and didn't grow on kanamycin, ampicilin, chloramphenicol medium. We obtain strain MG1655Z1 Δfnr. |
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining FNR and BphR2 proteins
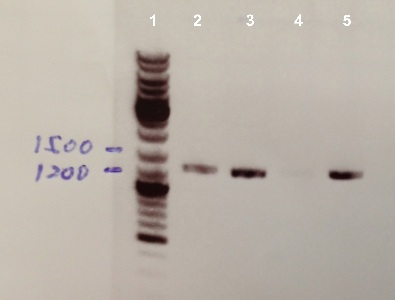
1 - Electrophoresis of PCR Colony of FNR, RBS-FNR and RBS-BphR2
Expect sizes :
- FNR : 1096 bp
- RBS-FNR : 1014 bp
- RBS-BphR2 : 1469 bp
|
We obtained fragments at the right size for FNR and RBS-FNR. The Gisbon assembly of 08/26/13 was good. |
| Previous day | Back to calendar | Next day |
 "
"