Team:TU-Eindhoven/AnearobicTesting
From 2013.igem.org
(→Fluorescence Measurements) |
(→Discussion and Conclusions) |
||
| (57 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:TU-Eindhoven/Template:Base}} | {{:Team:TU-Eindhoven/Template:Base}} | ||
{{:Team:TU-Eindhoven/Template:MenuBar}} | {{:Team:TU-Eindhoven/Template:MenuBar}} | ||
| + | |||
| + | =Anaerobic Testing of the FNR Promoter= | ||
== Abstract == | == Abstract == | ||
| - | + | When the bacteria have reached the tumor zone, the CEST contrast proteins should be expressed. A characteristic of tumorous areas is the low oxygen concentration, so when the proteins can be expressed if they sense a hypoxic environment, tumors can be visualized. Hereto production of the contrast generating proteins chosen for this experiment will be induced by triggering an FNR promoter, making use of hypoxic conditions. The chosen proteins are all Arginine and Lysine rich, which will ensure that CEST contrast becomes visible upon measurement with MRI machines, making bacterial protein expression a possible tumor imaging mechanism. Testing the promoter under hypoxic conditions it was revealed that no proteins were expressed in BL21 bacteria, but EGFP '''was''' produced in the XL-1 Blue and MG1655 strains. The XL1-Blue E.coli strain appears to be the best strain for the anaerobically induction of CEST protein expression, due to an increase in activity seen during the promoter experiments. | |
| - | + | == Introduction == | |
| - | Within the | + | Within the scope of this project, where bacteria are being used to express contrast proteins within tumor environments, the promoter had to be tested in similar conditions as to those in which tumor cells are present. Near and in tumors hypoxic (oxygen depleted) environments can be found. This means that the bacteria will need to express their proteins under hypoxic conditions, when tumor imaging is desired. The proteins that will be expressed are Arginine and Lysine rich proteins, which can give the proteins the feature of generating CEST contrast. As a proof of concept, the promoter that has been chosen (an FNR promoter, which is known to induce transcription when the environment has a low oxygen concentrations, in particular for metabolic cell activities), has to be tested under hypoxic conditions. |
| - | + | === Construct Design === | |
| - | The construct was designed as shown below. | + | The construct was designed as shown below. The design was achieved by incorporating a number of predetermined specifications: An oxygen sensitive promoter was needed, which is explained in more detail below (see: ''FNR''). To allow for purification of the proteins after expression, a His-Tag was included in front of the protein and a Thrombine cleavage site for cutting off the His-Tag after purification. Since the His-Tag will not be removed, the Thrombine cleavage site is not essential, but to reduce the number of differences between the anaerobic and aerobic sequences, a Thrombine cleavage site was inserted. Then the sequence for the CEST proteins/EGFP was placed into the construct. A spacer sequence and a terminator were placed behind the protein sequence to ensure correct termination of the protein expression. |
| - | For more information about the construct design, see [ | + | For more information about the construct design, see [[Team:TU-Eindhoven/Preparation | Construct Design]] and [[Team:TU-Eindhoven/Chasis | Chassis]]. |
{{:Team:TU-Eindhoven/Template:ImageList}} | {{:Team:TU-Eindhoven/Template:ImageList}} | ||
{{:Team:TU-Eindhoven/Template:Float | position=left | size=6 }} | {{:Team:TU-Eindhoven/Template:Float | position=left | size=6 }} | ||
| Line 17: | Line 19: | ||
{{:Team:TU-Eindhoven/Template:ImageListEnd}} | {{:Team:TU-Eindhoven/Template:ImageListEnd}} | ||
| - | + | === FNR === | |
| - | Since anaerobic expression is desired, a FNR promoter was inserted in front of the sequence for the desired protein. The promoter design was taken from research by Barnard et al. For more information about promoter design, see [ | + | Since anaerobic expression is desired, a FNR promoter was inserted in front of the sequence for the desired protein. The promoter design was taken from research by Barnard et al. For more information about promoter design, see [[Team:TU-Eindhoven/FNRPromotor | FNR Promoter]]. The chosen promoter had an 1.8 times higher activity than the most commonly used single binding site FNR promoter. The FNR promoter is regulated by the fumurate and nitrate reduction (FNR) protein. This is a transcription activator, that can control the expression of genes when oxygen concentrations are low. The inactive state of the FNR protein can acquire a [4Fe-4S]2+ cluster, subsequently it can form dimers containing [2Fe-2S]<sup>2+</sup> clusters. These dimers are the active transcription factors. Oxygen destabilizes these dimerized iron-sulfur-clusters which results in an inactive state of the transcription factor. For more information about the FNR dynamics, see [[Team:TU-Eindhoven/ODEModel | FNR Dynamics]]. |
| - | + | == Methods and Results == | |
| - | + | === Cloning === | |
| - | Once the pUC57 | + | Once the pUC57-simple constructs were delivered, we transformed them into NB bacteria for amplification of the vectors. Once amplified, a digestion on the constructs as well as on the pBR322 vector was performed with EcoRI and HindIII restriction enzymes to cut out the entire sequence (from promoter up to and including the terminator) out of the pUC57-simple construct. The pBR322 vector was chosen since it contains no promoter making it easier to test the FNR promoter. Once the pUC57-simple constructs as well as pBR322 vector were digested a gel extraction was performed to retain the length constructs. |
| - | + | Subsequently a ligation was performed to implant the complete sequences from the pUC57-simple constructs into the pBR322 vector. After ligation the constructs were plated with ampicilin antibiotics (for which resistance is introduced by the pBR322 vector) and grown overnight to create colonies. To check whether ligation had succeeded a colony PCR procedure was performed and the results were loaded on an agarose gel: | |
{{:Team:TU-Eindhoven/Template:ImageList}} | {{:Team:TU-Eindhoven/Template:ImageList}} | ||
| Line 34: | Line 36: | ||
{{:Team:TU-Eindhoven/Template:ImageListEnd}} | {{:Team:TU-Eindhoven/Template:ImageListEnd}} | ||
| - | + | Based on the above gels the following constructs were chosen to continue experimentation with: (Pro1(1,2), 1PJN(1,2), 1ETF(3,4), P(RG)(1,2), P(RS)(1,5), EGFP(1,2), 1G70(3,5), P(TK)(2,3), P(KS)(1,3). Small cultures were created, again with ampicilin, and grown overnight. To retain the vectors, a miniprep procedure was performed, the product of which was sequenced allowing us to show that each of the constructs was correctly implemented. | |
| + | The ligated vectors were then transformed into BL21 bacteria as preparation for protein expression. | ||
| - | + | === Protein Expression === | |
| - | + | For anaerobic protein expression a BioReactor, wherein anaerobic conditions can be reached by creating a flow of nitrogen through the culture, was used. | |
| + | First the ligated vectors were transformed into BL21 bacteria by heat shocking the bacteria. To test the FNR promoter the pBR322-EGFP construct was used, since EGFP can be measured with ease. 4L of LB medium was prepared and autoclaved within the vessel. This was subsequently inoculated with the bacteria, which were then grown aerobically until the optical density was approximately 0.6. Upon reaching the optical density of 0.6 the air supply was turned off and the vessel was continually flooded with Nitrogen ensuring an anaerobic environment. At several moments a sample was taken to follow the EGFP production curve. During the entire experiment the culture temperature was kept constant at 37 °C (equal to body temperature in which the bacteria should be able to function). | ||
| - | + | Upon analysis it was revealed that no EGFP had been produced. A possible explanation for this problem could be a mutation in the FNR coding gene within BL21 bacteria. This would mean that FNR production would not occur upon the bacteria entering an anaerobic environment. This was later confirmed by further research{{:Team:TU-Eindhoven/Template:Ref | id=NoFNRBL21 | author=Constanze Pinske et al| title=Metabolic Deficiences Revealed in the Biotechnologically Important Model Bacterium Escherichia coli BL21(DE3) | journal=PloS one | edition=6.8 | pages=e22830 | year=2011 }}. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | Since protein expression in BL21 bacteria was not | + | Since protein expression in BL21 bacteria was not successful, the pBR322-EGFP vector was then transformed, by heat shocking, into XL-1 Blue bacteria and by electroporation into an MG1655 strain, of which the latter was kindly provided by the University of Groningen. The same procedure for the anaerobic protein expression as was executed for the BL21 was now performed for the XL1-Blue and the MG1655 strain. In {{:Team:TU-Eindhoven/Template:Figure | id=SDSgelXL1blue }} and {{:Team:TU-Eindhoven/Template:Figure | id=SDSgelMG1655 }} the gel analyses of the BugBustered samples from the anaerobic induced EGFP expression are shown. |
| - | After His-Tag purification of the samples, another 12% SDS gel analysis was performed. From the XL1-Blue samples the | + | After His-Tag purification of the samples, another 12% SDS gel analysis was performed. From the XL1-Blue samples the whole time sequence that was obtained from the Biofermentor was purified, since the unpurified gel clearly showed increasing intensity of some bands. There is a not so clear band at the height about 29 kDa, which could indicate that EGFP was expressed. For the MG1655 samples only samples 1, 8 and 9 were taken to see whether there was an increase in intensity (this gel was less promising than the XL1-Blue samples). |
{{:Team:TU-Eindhoven/Template:ImageList}} | {{:Team:TU-Eindhoven/Template:ImageList}} | ||
| Line 57: | Line 57: | ||
{{:Team:TU-Eindhoven/Template:ImageListEnd}} | {{:Team:TU-Eindhoven/Template:ImageListEnd}} | ||
| - | |||
| - | |||
| - | ==== Fluorescence Measurements | + | The gel with the purified samples is shown in {{:Team:TU-Eindhoven/Template:Figure | id=SDSgelXL1blueMG1655pur }}. For the XL1-Blue samples there is a band around 50 kDa that increases in intensity. There is also a less intense band that might increase in intensity at around 30 kDa. This could indicate that EGFP, of which the molecular weight is 30kDa, is being expressed. Since the concentrations measured using nandrop after purification were very low, this could explain why the intensity is not that high. For the MG1655 a clear band is located around 45 kDa, which seems to correspond with the bands seen before on the unpurified gel, but this does not give us any information about EGFP expression. |
| - | From the purified | + | By cross referencing these gels with previous gels from other experiments we were able to propose with high certainty that the thicker bands at 50kDA were native proteins being expressed naturally in anaerobic environments. A good follow up experiment could be to find out which proteins these are and to investigate their expression mechanism, although this is most definitely beyond the scope of our current experiment. |
| + | |||
| + | {{:Team:TU-Eindhoven/Template:ImageList}} | ||
| + | {{:Team:TU-Eindhoven/Template:Float | position=left | size=8 }} | ||
| + | {{:Team:TU-Eindhoven/Template:Image | filename=SDSgelXL1blueMG1655pur.jpg}} | ||
| + | {{:Team:TU-Eindhoven/Template:FloatEnd | caption=12% SDS gel with the purified samples of the XL1-Blue and the MG1655 anaerobic expressions of EGFP.| id=SDSgelXL1blueMG1655pur }} | ||
| + | {{:Team:TU-Eindhoven/Template:ImageListEnd}} | ||
| + | |||
| + | ====Repeat Experiments==== | ||
| + | To further test the FNR promoter and therefore the expression of EGFP, the above mentioned experiments were repeated two further times. The first of these repeats was equal to the previous expression experiments, where the oxygen concentration was kept at 0%. The second differed in this fact by maintaining a constant oxygen concentration of 5%. During both these expressions the optical density of the medium was recorded each time a sample was taken, which would allow for a more accurate representation of the EGFP expression per bacterial cell. Aside from these minor differences the experiments were kept equal to one another and the same processing steps were performed upon completion. (It should be noted that the experiments were performed using the Xl-1-Blue E.coli strain as this strain showed the most promising results the previous time around. | ||
| + | |||
| + | Having taken the samples during the expression experiment they were spun down and purified. This purified sample was then loaded onto a 12% SDS gel for analysis. | ||
| + | |||
| + | {{:Team:TU-Eindhoven/Template:ImageList}} | ||
| + | {{:Team:TU-Eindhoven/Template:Float | position=left | size=8 }} | ||
| + | {{:Team:TU-Eindhoven/Template:Image | filename=AnaerobicEGFPExpressionPurifiedSDS.png}} | ||
| + | {{:Team:TU-Eindhoven/Template:FloatEnd | caption= The columns labelled C- and C+ are respectively a negative and positive control. The negative control was obtained by purifying a sample of an aerobic culture containing the same bacteria. The positive control was a known EGFP protein (which has a higher height due its binding to the CNA35 protein. There are also two sets of samples with the names t1 through to t6. The first of these come from the repeat experiment at 0% Oxygen and the second set from the repeat experiment at 5% Oxygen. | id=SDSgelPurified0andprocent }} | ||
| + | {{:Team:TU-Eindhoven/Template:ImageListEnd}} | ||
| + | |||
| + | Looking at {{:Team:TU-Eindhoven/Template:Figure | id=SDSgelPurified0andprocent }} the thick band of proteins at approximately 60kDa is again clearly visible. Again the band where EGFP is to be expected has been outlined and once again the bands seen there are of very low intensity. Looking at these results it would appear that they are equal to the results of the previously performed expression. | ||
| + | In an attempt to enhance the EGFP bands, and to show without doubt that these vague bands are in fact EGFP, it was decided to perform a Western Blot. | ||
| + | |||
| + | The Western Blot was performed by first running a 12% SDS gel loaded with the same samples as were loaded on the SDS gel shown in {{:Team:TU-Eindhoven/Template:Figure | id=SDSgelPurified0andprocent }}. This gel was then blotted onto a nitrocellulose membrane. After blotting for 60 minutes at 100V the membrane was washed in a PBS/Tween solution containing 5% milk powder. Hereafter the membrane was washed, now with a PBS/Tween solution containing 0.5% milk powder and EGFP binding antibodies. After washing this antibody solution off the membrane with PBS/Tween solution, another 0.5% milk powder PBS/Tween solution now with the according secondary antibody was poured over the membrane. Finally the membrane was washed with pure PBS/Tween again before being stained with TMB (tetramethylbenzidine) solution. This revealed the EGFP bands which were still to be found on the membrane. The stained membrane can be seen below in {{:Team:TU-Eindhoven/Template:Figure | id=WesternBlot }}. | ||
| + | |||
| + | {{:Team:TU-Eindhoven/Template:ImageList}} | ||
| + | {{:Team:TU-Eindhoven/Template:Float | position=left | size=8 }} | ||
| + | {{:Team:TU-Eindhoven/Template:Image | filename=WesternBlot.png}} | ||
| + | {{:Team:TU-Eindhoven/Template:FloatEnd | caption= The same loading system was used for the Western Blot as was used to show the purified samples from the repeat experiments. | id=WesternBlot }} | ||
| + | {{:Team:TU-Eindhoven/Template:ImageListEnd}} | ||
| + | |||
| + | When looking at {{:Team:TU-Eindhoven/Template:Figure | id=WesternBlot }} it is clear that the bands of low intensity at approximately 29kDa do indeed correlate with the EGFP protein. The higher and thick intensity bands have completely disappeared, meaning they were not bound by the EGFP specific antibodies. What is of concern is that there is an EGFP band visible in the negative control. This sample was taken from an aerobic expression, and therefore no EGFP was expected. | ||
| + | |||
| + | === Fluorescence Measurements === | ||
| + | From the purified protein solutions, samples were taken for fluorescence analysis. EGFP emission spectra were desired to have an increased intensity when the samples were taken at later points in time. From this we would be able to conclude that more EGFP was being expressed as the bacteria spent more time in anaerobic conditions. The samples from MG1655 did show the right emission spectra, but there was no increase in intensity between the samples. That the signal was merely caused by scattering was excluded since a shift in excitation wavelength did not result in a shift in emission spectrum. The samples of the XL1-Blue expression, however, '''did''' show an increase in intensity. The results are shown in {{:Team:TU-Eindhoven/Template:Figure | id=Fluorescencemeasurements }}. Here scattering was also excluded. The fluorescence intensities of the peaks at λ = 508 nm are shown in {{:Team:TU-Eindhoven/Template:Figure | id=Emissionpeaks }}. A comment on these measurements is that the gain was relatively high (187), which is a result of the low concentrations of EGFP formed. | ||
{{:Team:TU-Eindhoven/Template:ImageList}} | {{:Team:TU-Eindhoven/Template:ImageList}} | ||
| Line 72: | Line 103: | ||
{{:Team:TU-Eindhoven/Template:ImageListEnd}} | {{:Team:TU-Eindhoven/Template:ImageListEnd}} | ||
| - | In | + | In {{:Team:TU-Eindhoven/Template:Figure | id=Emissionpeaks }}, the second sample shows a decrease in intensity. This can be caused by the switch from aerobic to anaerobic metabolism so that the EGFP that was produced can be degraded until the bacteria are fully switched to anaerobic metabolism. Then the peaks increase, caused by higher concentrations of EGFP produced anaerobically by the bacteria. Another reason for this could be that the bacteria continued to grow and that the produced EGFP is background expression. |
| - | The intensity of the last sample | + | The intensity of the last sample showed decreased intensity with respects to the peak of around 18-19 hours. This can also be caused by degradation of the produced proteins. |
| + | |||
| + | ====Repeat Experiments==== | ||
| + | As repeat experiments had been performed, where samples of EGFP expression had been taken and purified it would also be possible to perform fluorescence experiments. These would be performed in the same way as was done previously. When analyzing the results of these measurements however it was now possible to normalize each of the emission intensities with the Optical Density of the sample. In this way a more accurate representation of the EGFP expression per bacterium. | ||
| + | |||
| + | {{:Team:TU-Eindhoven/Template:ImageList}} | ||
| + | {{:Team:TU-Eindhoven/Template:Float | position=left | size=6 }} | ||
| + | {{:Team:TU-Eindhoven/Template:Image | filename=FluorAnalysis-22okt.png}} | ||
| + | {{:Team:TU-Eindhoven/Template:FloatEnd | caption=Emission intensity peaks per sample for anaerobic expression at 0% Oxygen| id=Emissionpeaks0 }} | ||
| + | {{:Team:TU-Eindhoven/Template:Float | position=right | size=6 }} | ||
| + | {{:Team:TU-Eindhoven/Template:Image | filename=FluorAnalysis-23okt2.png}} | ||
| + | {{:Team:TU-Eindhoven/Template:FloatEnd | caption=Emission intensity peaks per sample for anaerobic expression at 5% Oxygen| id=Emissionpeaks5 }} | ||
| + | {{:Team:TU-Eindhoven/Template:ImageListEnd}} | ||
| + | |||
| + | Looking at {{:Team:TU-Eindhoven/Template:Figure | id=Emissionpeaks0 }} it is notable that, unlike the former anaerobic expression experiments the intensity begins to decrease within a few hours of the anaerobic expression, instead of only after 20+ hours as seen previously. {{:Team:TU-Eindhoven/Template:Figure | id=Emissionpeaks5 }} however shows that the intensity continues to increase dramatically even after 20 hours in anaerobic conditions. | ||
| - | + | == Discussion and Conclusions == | |
| - | SDS gel analysis of the unpurified samples from the XL1-Blue culture indicate that there are several | + | SDS gel analysis of the unpurified samples from the XL1-Blue culture indicate that there are several proteins showing increased expression when the samples are left in anaerobic conditions for longer periods of time. Here the bands of interest were at the height of 45-50 kDa and around 30 kDa. After purifying by the His-Tag, the SDS gel did not contain the clear bands which it had on the unpurified gel, but there are bands at the same height (around 45-50 kDa) and bands around 29 kDa, which do show a slight increase in intensity, even if the relative intensity is marginal. Performing a Western blot on these purified samples does however give a clear indication of EGFP at the expected height of 29kDa. All other bands seen on the purified and unpurified SDS gels had disappeared leaving only the EGFP bands visible, and showing without doubt that EGFP had been expressed. Fluorescence measurements of the purified samples also indicate that there is indeed EGFP expression and that the intensity increases when the cultures are exposed to the anaerobic environment. Here we have to place the comment that not all the experiments revealed the same results and that there was some variation in the EGFP expression. This could be caused by degradation of the proteins. Scattering was excluded by testing other excitation wavelengths each of which resulted in the same emission spectra. |
| - | + | It can be concluded that EGFP is expressed under the regulation of oxygen concentration, even though the concentrations are rather low resulting in low intensity bands on the purified SDS gel and a high gain for the fluorescence measurements. The low concentrations were to be expected however as the EGFP was not being over expressed and the samples taken form the BioReactor were a mere 20mL. | |
For the SDS gel of the unpurified MG1655 samples we saw some bands that indicate an increase of protein expression. These bands also show up on the purified samples SDS gel. The bands are at the same height as the bands on the unpurified gels, so they correspond. The fluorescence measurements for the purified MG1655 samples showed the correct emission spectra for EGFP. The spectra for the samples that were longer exposed did not have an increased intensity. Scattering was excluded by taking other excitation wavelengths, which resulted in the same emission spectra. | For the SDS gel of the unpurified MG1655 samples we saw some bands that indicate an increase of protein expression. These bands also show up on the purified samples SDS gel. The bands are at the same height as the bands on the unpurified gels, so they correspond. The fluorescence measurements for the purified MG1655 samples showed the correct emission spectra for EGFP. The spectra for the samples that were longer exposed did not have an increased intensity. Scattering was excluded by taking other excitation wavelengths, which resulted in the same emission spectra. | ||
| - | Overall we can conclude that the FNR promoter does work and EGFP is produced anaerobically. | + | Overall we can conclude that the FNR promoter does work and EGFP is produced anaerobically. We do however recommend the XL-1-Blue bacterial strain for the expression of proteins under influence of the FNR promoter over the MG1655 E.coli strain. Going off on the repeat experiments, it can also be mentioned that expression at slightly higher Oxygen concentrations is also possible, and could even provide higher EGFP expression. |
| + | ==References== | ||
| + | {{:Team:TU-Eindhoven/Template:RefList}} | ||
{{:Team:TU-Eindhoven/Template:BaseFooter}} | {{:Team:TU-Eindhoven/Template:BaseFooter}} | ||
| + | {{:Team:TU-Eindhoven/Template:Sponsors}} | ||
{{:Team:TU-Eindhoven/Template:UseFigures}} | {{:Team:TU-Eindhoven/Template:UseFigures}} | ||
| + | {{:Team:TU-Eindhoven/Template:UseReferencing}} | ||
{{:Team:TU-Eindhoven/Template:SetTitle | menu=wetlab | page=Anaerobic FNR Testing}} | {{:Team:TU-Eindhoven/Template:SetTitle | menu=wetlab | page=Anaerobic FNR Testing}} | ||
{{:Team:TU-Eindhoven/Template:SetHeader | nr=1}} | {{:Team:TU-Eindhoven/Template:SetHeader | nr=1}} | ||
Latest revision as of 14:43, 26 October 2013



Contents |
Anaerobic Testing of the FNR Promoter
Abstract
When the bacteria have reached the tumor zone, the CEST contrast proteins should be expressed. A characteristic of tumorous areas is the low oxygen concentration, so when the proteins can be expressed if they sense a hypoxic environment, tumors can be visualized. Hereto production of the contrast generating proteins chosen for this experiment will be induced by triggering an FNR promoter, making use of hypoxic conditions. The chosen proteins are all Arginine and Lysine rich, which will ensure that CEST contrast becomes visible upon measurement with MRI machines, making bacterial protein expression a possible tumor imaging mechanism. Testing the promoter under hypoxic conditions it was revealed that no proteins were expressed in BL21 bacteria, but EGFP was produced in the XL-1 Blue and MG1655 strains. The XL1-Blue E.coli strain appears to be the best strain for the anaerobically induction of CEST protein expression, due to an increase in activity seen during the promoter experiments.
Introduction
Within the scope of this project, where bacteria are being used to express contrast proteins within tumor environments, the promoter had to be tested in similar conditions as to those in which tumor cells are present. Near and in tumors hypoxic (oxygen depleted) environments can be found. This means that the bacteria will need to express their proteins under hypoxic conditions, when tumor imaging is desired. The proteins that will be expressed are Arginine and Lysine rich proteins, which can give the proteins the feature of generating CEST contrast. As a proof of concept, the promoter that has been chosen (an FNR promoter, which is known to induce transcription when the environment has a low oxygen concentrations, in particular for metabolic cell activities), has to be tested under hypoxic conditions.
Construct Design
The construct was designed as shown below. The design was achieved by incorporating a number of predetermined specifications: An oxygen sensitive promoter was needed, which is explained in more detail below (see: FNR). To allow for purification of the proteins after expression, a His-Tag was included in front of the protein and a Thrombine cleavage site for cutting off the His-Tag after purification. Since the His-Tag will not be removed, the Thrombine cleavage site is not essential, but to reduce the number of differences between the anaerobic and aerobic sequences, a Thrombine cleavage site was inserted. Then the sequence for the CEST proteins/EGFP was placed into the construct. A spacer sequence and a terminator were placed behind the protein sequence to ensure correct termination of the protein expression. For more information about the construct design, see Construct Design and Chassis.
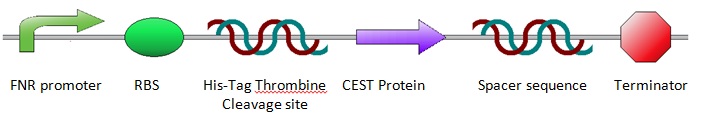
FNR
Since anaerobic expression is desired, a FNR promoter was inserted in front of the sequence for the desired protein. The promoter design was taken from research by Barnard et al. For more information about promoter design, see FNR Promoter. The chosen promoter had an 1.8 times higher activity than the most commonly used single binding site FNR promoter. The FNR promoter is regulated by the fumurate and nitrate reduction (FNR) protein. This is a transcription activator, that can control the expression of genes when oxygen concentrations are low. The inactive state of the FNR protein can acquire a [4Fe-4S]2+ cluster, subsequently it can form dimers containing [2Fe-2S]2+ clusters. These dimers are the active transcription factors. Oxygen destabilizes these dimerized iron-sulfur-clusters which results in an inactive state of the transcription factor. For more information about the FNR dynamics, see FNR Dynamics.
Methods and Results
Cloning
Once the pUC57-simple constructs were delivered, we transformed them into NB bacteria for amplification of the vectors. Once amplified, a digestion on the constructs as well as on the pBR322 vector was performed with EcoRI and HindIII restriction enzymes to cut out the entire sequence (from promoter up to and including the terminator) out of the pUC57-simple construct. The pBR322 vector was chosen since it contains no promoter making it easier to test the FNR promoter. Once the pUC57-simple constructs as well as pBR322 vector were digested a gel extraction was performed to retain the length constructs. Subsequently a ligation was performed to implant the complete sequences from the pUC57-simple constructs into the pBR322 vector. After ligation the constructs were plated with ampicilin antibiotics (for which resistance is introduced by the pBR322 vector) and grown overnight to create colonies. To check whether ligation had succeeded a colony PCR procedure was performed and the results were loaded on an agarose gel:
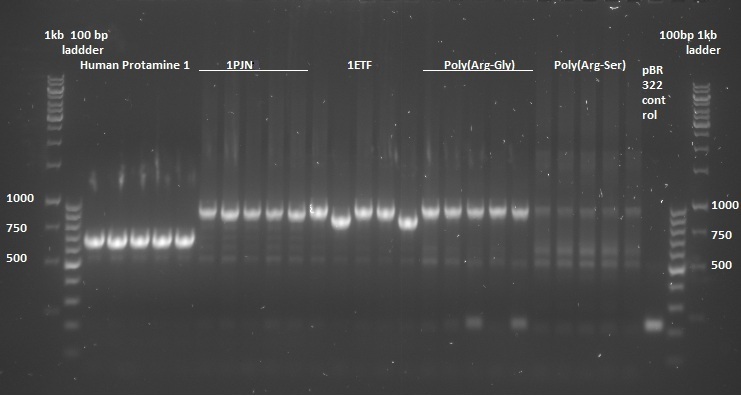
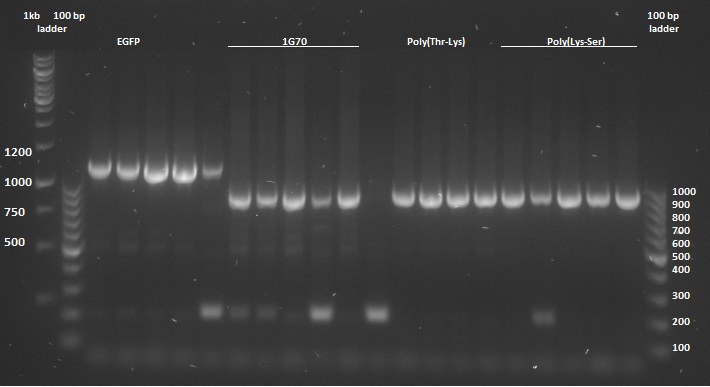
Based on the above gels the following constructs were chosen to continue experimentation with: (Pro1(1,2), 1PJN(1,2), 1ETF(3,4), P(RG)(1,2), P(RS)(1,5), EGFP(1,2), 1G70(3,5), P(TK)(2,3), P(KS)(1,3). Small cultures were created, again with ampicilin, and grown overnight. To retain the vectors, a miniprep procedure was performed, the product of which was sequenced allowing us to show that each of the constructs was correctly implemented. The ligated vectors were then transformed into BL21 bacteria as preparation for protein expression.
Protein Expression
For anaerobic protein expression a BioReactor, wherein anaerobic conditions can be reached by creating a flow of nitrogen through the culture, was used. First the ligated vectors were transformed into BL21 bacteria by heat shocking the bacteria. To test the FNR promoter the pBR322-EGFP construct was used, since EGFP can be measured with ease. 4L of LB medium was prepared and autoclaved within the vessel. This was subsequently inoculated with the bacteria, which were then grown aerobically until the optical density was approximately 0.6. Upon reaching the optical density of 0.6 the air supply was turned off and the vessel was continually flooded with Nitrogen ensuring an anaerobic environment. At several moments a sample was taken to follow the EGFP production curve. During the entire experiment the culture temperature was kept constant at 37 °C (equal to body temperature in which the bacteria should be able to function).
Upon analysis it was revealed that no EGFP had been produced. A possible explanation for this problem could be a mutation in the FNR coding gene within BL21 bacteria. This would mean that FNR production would not occur upon the bacteria entering an anaerobic environment. This was later confirmed by further researchNoFNRBL21Constanze Pinske et al, Metabolic Deficiences Revealed in the Biotechnologically Important Model Bacterium Escherichia coli BL21(DE3). PloS one 6.8, e22830 (2011).
Since protein expression in BL21 bacteria was not successful, the pBR322-EGFP vector was then transformed, by heat shocking, into XL-1 Blue bacteria and by electroporation into an MG1655 strain, of which the latter was kindly provided by the University of Groningen. The same procedure for the anaerobic protein expression as was executed for the BL21 was now performed for the XL1-Blue and the MG1655 strain. In and the gel analyses of the BugBustered samples from the anaerobic induced EGFP expression are shown. After His-Tag purification of the samples, another 12% SDS gel analysis was performed. From the XL1-Blue samples the whole time sequence that was obtained from the Biofermentor was purified, since the unpurified gel clearly showed increasing intensity of some bands. There is a not so clear band at the height about 29 kDa, which could indicate that EGFP was expressed. For the MG1655 samples only samples 1, 8 and 9 were taken to see whether there was an increase in intensity (this gel was less promising than the XL1-Blue samples).
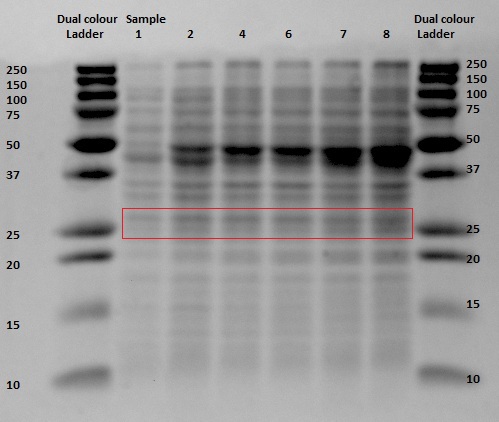
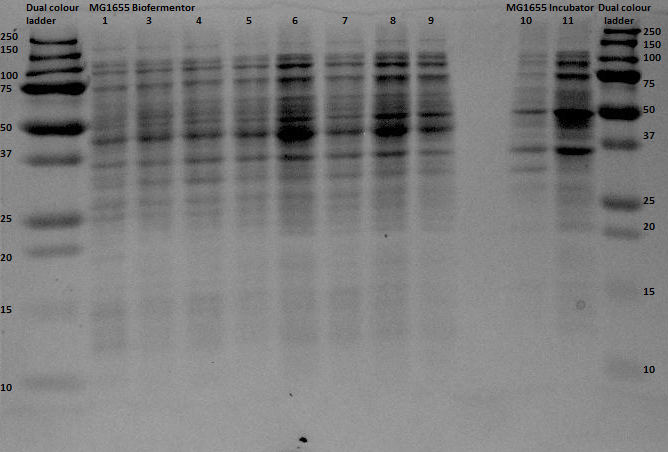
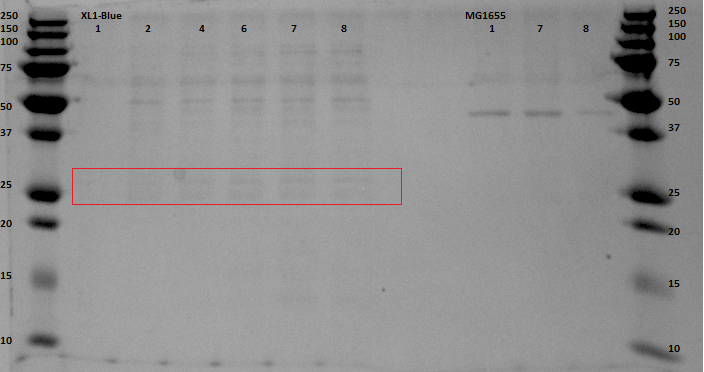
The gel with the purified samples is shown in . For the XL1-Blue samples there is a band around 50 kDa that increases in intensity. There is also a less intense band that might increase in intensity at around 30 kDa. This could indicate that EGFP, of which the molecular weight is 30kDa, is being expressed. Since the concentrations measured using nandrop after purification were very low, this could explain why the intensity is not that high. For the MG1655 a clear band is located around 45 kDa, which seems to correspond with the bands seen before on the unpurified gel, but this does not give us any information about EGFP expression.
By cross referencing these gels with previous gels from other experiments we were able to propose with high certainty that the thicker bands at 50kDA were native proteins being expressed naturally in anaerobic environments. A good follow up experiment could be to find out which proteins these are and to investigate their expression mechanism, although this is most definitely beyond the scope of our current experiment.
Repeat Experiments
To further test the FNR promoter and therefore the expression of EGFP, the above mentioned experiments were repeated two further times. The first of these repeats was equal to the previous expression experiments, where the oxygen concentration was kept at 0%. The second differed in this fact by maintaining a constant oxygen concentration of 5%. During both these expressions the optical density of the medium was recorded each time a sample was taken, which would allow for a more accurate representation of the EGFP expression per bacterial cell. Aside from these minor differences the experiments were kept equal to one another and the same processing steps were performed upon completion. (It should be noted that the experiments were performed using the Xl-1-Blue E.coli strain as this strain showed the most promising results the previous time around.
Having taken the samples during the expression experiment they were spun down and purified. This purified sample was then loaded onto a 12% SDS gel for analysis.

Looking at the thick band of proteins at approximately 60kDa is again clearly visible. Again the band where EGFP is to be expected has been outlined and once again the bands seen there are of very low intensity. Looking at these results it would appear that they are equal to the results of the previously performed expression. In an attempt to enhance the EGFP bands, and to show without doubt that these vague bands are in fact EGFP, it was decided to perform a Western Blot.
The Western Blot was performed by first running a 12% SDS gel loaded with the same samples as were loaded on the SDS gel shown in . This gel was then blotted onto a nitrocellulose membrane. After blotting for 60 minutes at 100V the membrane was washed in a PBS/Tween solution containing 5% milk powder. Hereafter the membrane was washed, now with a PBS/Tween solution containing 0.5% milk powder and EGFP binding antibodies. After washing this antibody solution off the membrane with PBS/Tween solution, another 0.5% milk powder PBS/Tween solution now with the according secondary antibody was poured over the membrane. Finally the membrane was washed with pure PBS/Tween again before being stained with TMB (tetramethylbenzidine) solution. This revealed the EGFP bands which were still to be found on the membrane. The stained membrane can be seen below in .
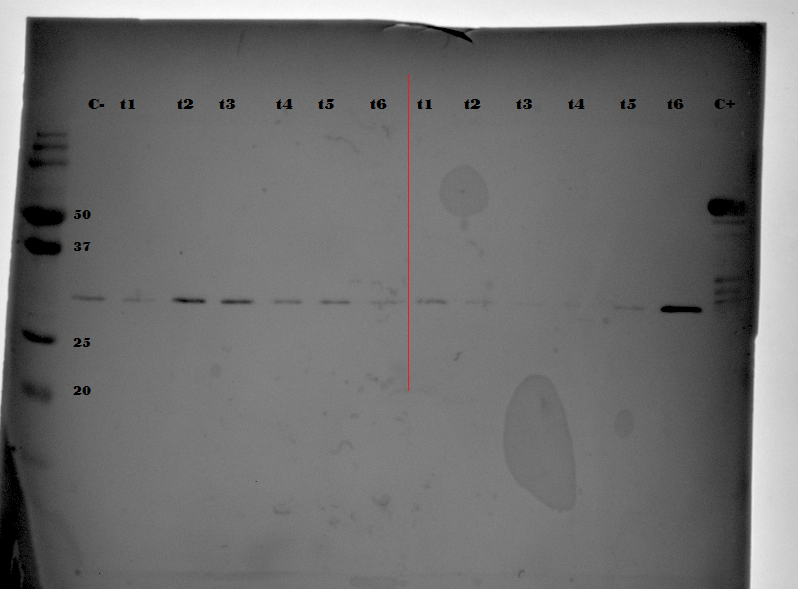
When looking at it is clear that the bands of low intensity at approximately 29kDa do indeed correlate with the EGFP protein. The higher and thick intensity bands have completely disappeared, meaning they were not bound by the EGFP specific antibodies. What is of concern is that there is an EGFP band visible in the negative control. This sample was taken from an aerobic expression, and therefore no EGFP was expected.
Fluorescence Measurements
From the purified protein solutions, samples were taken for fluorescence analysis. EGFP emission spectra were desired to have an increased intensity when the samples were taken at later points in time. From this we would be able to conclude that more EGFP was being expressed as the bacteria spent more time in anaerobic conditions. The samples from MG1655 did show the right emission spectra, but there was no increase in intensity between the samples. That the signal was merely caused by scattering was excluded since a shift in excitation wavelength did not result in a shift in emission spectrum. The samples of the XL1-Blue expression, however, did show an increase in intensity. The results are shown in . Here scattering was also excluded. The fluorescence intensities of the peaks at λ = 508 nm are shown in . A comment on these measurements is that the gain was relatively high (187), which is a result of the low concentrations of EGFP formed.
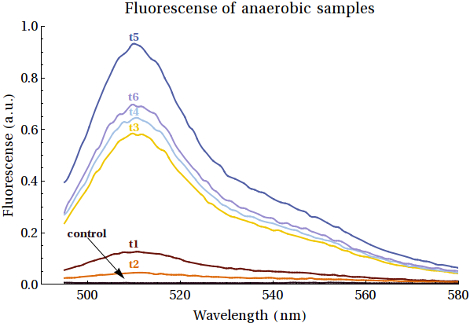
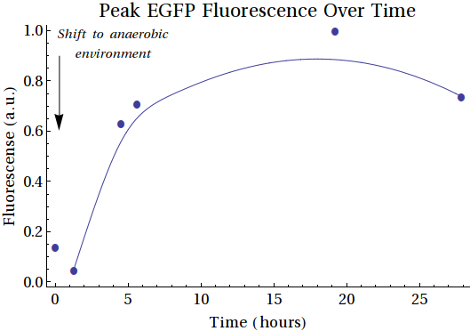
In , the second sample shows a decrease in intensity. This can be caused by the switch from aerobic to anaerobic metabolism so that the EGFP that was produced can be degraded until the bacteria are fully switched to anaerobic metabolism. Then the peaks increase, caused by higher concentrations of EGFP produced anaerobically by the bacteria. Another reason for this could be that the bacteria continued to grow and that the produced EGFP is background expression. The intensity of the last sample showed decreased intensity with respects to the peak of around 18-19 hours. This can also be caused by degradation of the produced proteins.
Repeat Experiments
As repeat experiments had been performed, where samples of EGFP expression had been taken and purified it would also be possible to perform fluorescence experiments. These would be performed in the same way as was done previously. When analyzing the results of these measurements however it was now possible to normalize each of the emission intensities with the Optical Density of the sample. In this way a more accurate representation of the EGFP expression per bacterium.
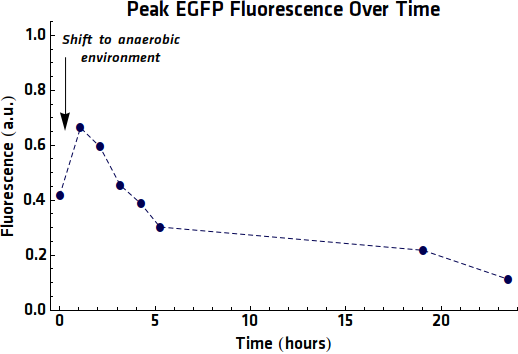
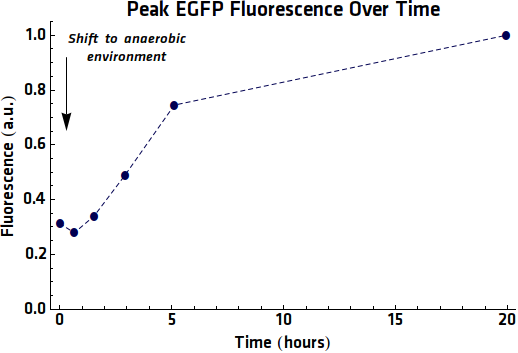
Looking at it is notable that, unlike the former anaerobic expression experiments the intensity begins to decrease within a few hours of the anaerobic expression, instead of only after 20+ hours as seen previously. however shows that the intensity continues to increase dramatically even after 20 hours in anaerobic conditions.
Discussion and Conclusions
SDS gel analysis of the unpurified samples from the XL1-Blue culture indicate that there are several proteins showing increased expression when the samples are left in anaerobic conditions for longer periods of time. Here the bands of interest were at the height of 45-50 kDa and around 30 kDa. After purifying by the His-Tag, the SDS gel did not contain the clear bands which it had on the unpurified gel, but there are bands at the same height (around 45-50 kDa) and bands around 29 kDa, which do show a slight increase in intensity, even if the relative intensity is marginal. Performing a Western blot on these purified samples does however give a clear indication of EGFP at the expected height of 29kDa. All other bands seen on the purified and unpurified SDS gels had disappeared leaving only the EGFP bands visible, and showing without doubt that EGFP had been expressed. Fluorescence measurements of the purified samples also indicate that there is indeed EGFP expression and that the intensity increases when the cultures are exposed to the anaerobic environment. Here we have to place the comment that not all the experiments revealed the same results and that there was some variation in the EGFP expression. This could be caused by degradation of the proteins. Scattering was excluded by testing other excitation wavelengths each of which resulted in the same emission spectra. It can be concluded that EGFP is expressed under the regulation of oxygen concentration, even though the concentrations are rather low resulting in low intensity bands on the purified SDS gel and a high gain for the fluorescence measurements. The low concentrations were to be expected however as the EGFP was not being over expressed and the samples taken form the BioReactor were a mere 20mL.
For the SDS gel of the unpurified MG1655 samples we saw some bands that indicate an increase of protein expression. These bands also show up on the purified samples SDS gel. The bands are at the same height as the bands on the unpurified gels, so they correspond. The fluorescence measurements for the purified MG1655 samples showed the correct emission spectra for EGFP. The spectra for the samples that were longer exposed did not have an increased intensity. Scattering was excluded by taking other excitation wavelengths, which resulted in the same emission spectra.
Overall we can conclude that the FNR promoter does work and EGFP is produced anaerobically. We do however recommend the XL-1-Blue bacterial strain for the expression of proteins under influence of the FNR promoter over the MG1655 E.coli strain. Going off on the repeat experiments, it can also be mentioned that expression at slightly higher Oxygen concentrations is also possible, and could even provide higher EGFP expression.
References
 "
"



