Team:Paris Saclay/Notebook/July/15
From 2013.igem.org
(Difference between revisions)
(Created page with "='''Notebook : July 15'''= =='''Lab work'''== constructing <br> {| border="1" align="center" |<big>Previous week</big> |[[Team:Paris_Sac...") |
|||
| (15 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Team:Paris_Saclay/incl_debut_generique}} | ||
| + | |||
='''Notebook : July 15'''= | ='''Notebook : July 15'''= | ||
=='''Lab work'''== | =='''Lab work'''== | ||
| - | |||
| - | + | ==='''A - Aerobic/Anaerobic regulation system'''=== | |
| + | |||
| + | ===='''Objective : obtaining BBa_K1155000'''==== | ||
| + | |||
| + | ===='''1 - Sequensis analysis of BBa_K1155000'''==== | ||
| + | |||
| + | Abdou | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DE;" | | ||
| + | WE HAVE OUR FIRST BIOBRICK ! | ||
| + | The sequence analysis was good. We call our biobrick BBa_K1155000. | ||
| + | |} | ||
| + | |||
| + | ===='''Objective : obtaining BBa_K1155003, BBa_K1155007'''==== | ||
| + | |||
| + | ===='''1 - Liquid culture of BBa_B0015, BBa_B0017, BBa_I732019'''==== | ||
| + | |||
| + | Sheng, Zhou | ||
| + | |||
| + | {| | ||
| + | | style="border:1px solid black;padding:5px;background-color:#DE;" | | ||
| + | Tranformations of 07/12/13 works. We will do a liquid culture of them. | ||
| + | |} | ||
| + | |||
| + | We used 5 colonies of each plasmid. | ||
| + | |||
| + | |||
| + | ==='''B - PCB sensor system'''=== | ||
| + | |||
| + | ===='''Objective : obtaining BBa_K1155001, BBa_K1155002, BphR2'''==== | ||
| + | |||
| + | ===='''1 - Digestion of BBa_K1155001, BBa_K1155002 and BphR2 in pSB1C3 by EcoRI/PstI and SacII to check the size of fragments'''==== | ||
| + | |||
| + | Abdou | ||
| + | |||
| + | Used quantities : | ||
| + | |||
| + | * EcoRI/PstI : | ||
| + | ** DNA : 8µL | ||
| + | ** Buffer Orange : 1.6µL | ||
| + | ** EcoRI : 1µL | ||
| + | ** PstI : 1µL | ||
| + | ** H2O : 4.4µL | ||
| + | |||
| + | * SacII : | ||
| + | ** DNA : 8µL | ||
| + | ** Buffer Blue : 1.6µL | ||
| + | ** SacII : 1µL | ||
| + | ** H2O : 5.4µL | ||
| + | |||
| + | We let the digestion 1h30 at 37°C. | ||
| + | |||
| + | ===='''2 - PCR of digestion of BBa_K1155001, BBa_K1155002 and BphR2 in pSB1C3'''==== | ||
| + | |||
| + | Anaïs, Sheng, Zhou | ||
| + | |||
| + | Used quantities : | ||
| + | |||
| + | We mix our colonies in 20µL of H2O : | ||
| + | |||
| + | Used quantities : | ||
| + | * BphA1 or BphR1 or BphR2 : 2µL | ||
| + | * Mix : (it was divided in 2 tubes for each oligo combinaison with 23µL of mix in each tube) | ||
| + | ** VF or Pfnr_Up : 6µL | ||
| + | ** VR or Pfnr_Down or VR : 6µL | ||
| + | ** dNTP : 6µL | ||
| + | ** Buffer Dream Taq : 30µL | ||
| + | ** Dream Taq : 6µL | ||
| + | ** H2O : 246µL | ||
| + | |||
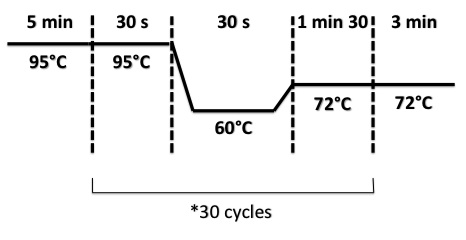
| + | PCR Program : | ||
| + | |||
| + | * BphR1 : | ||
| + | |||
| + | [[File:PsPCRR11507.jpg|400px]] | ||
| + | |||
| + | * BphA1, BphR2 : | ||
| + | |||
| + | [[File:PsPCRR2A11507.jpg|400px]] | ||
| + | |||
{| border="1" align="center" | {| border="1" align="center" | ||
|[[Team:Paris Saclay/Notebook/July/12|<big>Previous week</big>]] | |[[Team:Paris Saclay/Notebook/July/12|<big>Previous week</big>]] | ||
| Line 10: | Line 91: | ||
|[[Team:Paris_Saclay/Notebook|<big>Back to calendar</big>]] | |[[Team:Paris_Saclay/Notebook|<big>Back to calendar</big>]] | ||
| - | |[[Team:Paris Saclay/Notebook/July/16|<big> | + | |[[Team:Paris Saclay/Notebook/July/16|<big>Next day</big>]] |
|} | |} | ||
{{Team:Paris_Saclay/incl_fin}} | {{Team:Paris_Saclay/incl_fin}} | ||
Latest revision as of 23:59, 4 October 2013
Contents |
Notebook : July 15
Lab work
A - Aerobic/Anaerobic regulation system
Objective : obtaining BBa_K1155000
1 - Sequensis analysis of BBa_K1155000
Abdou
|
WE HAVE OUR FIRST BIOBRICK ! The sequence analysis was good. We call our biobrick BBa_K1155000. |
Objective : obtaining BBa_K1155003, BBa_K1155007
1 - Liquid culture of BBa_B0015, BBa_B0017, BBa_I732019
Sheng, Zhou
|
Tranformations of 07/12/13 works. We will do a liquid culture of them. |
We used 5 colonies of each plasmid.
B - PCB sensor system
Objective : obtaining BBa_K1155001, BBa_K1155002, BphR2
1 - Digestion of BBa_K1155001, BBa_K1155002 and BphR2 in pSB1C3 by EcoRI/PstI and SacII to check the size of fragments
Abdou
Used quantities :
- EcoRI/PstI :
- DNA : 8µL
- Buffer Orange : 1.6µL
- EcoRI : 1µL
- PstI : 1µL
- H2O : 4.4µL
- SacII :
- DNA : 8µL
- Buffer Blue : 1.6µL
- SacII : 1µL
- H2O : 5.4µL
We let the digestion 1h30 at 37°C.
2 - PCR of digestion of BBa_K1155001, BBa_K1155002 and BphR2 in pSB1C3
Anaïs, Sheng, Zhou
Used quantities :
We mix our colonies in 20µL of H2O :
Used quantities :
- BphA1 or BphR1 or BphR2 : 2µL
- Mix : (it was divided in 2 tubes for each oligo combinaison with 23µL of mix in each tube)
- VF or Pfnr_Up : 6µL
- VR or Pfnr_Down or VR : 6µL
- dNTP : 6µL
- Buffer Dream Taq : 30µL
- Dream Taq : 6µL
- H2O : 246µL
PCR Program :
- BphR1 :
- BphA1, BphR2 :
| Previous week | Back to calendar | Next day |
 "
"