Team:Duke/Modeling/1
From 2013.igem.org
Hyunsoo kim (Talk | contribs) |
Hyunsoo kim (Talk | contribs) |
||
| Line 7: | Line 7: | ||
== Cooperativity == | == Cooperativity == | ||
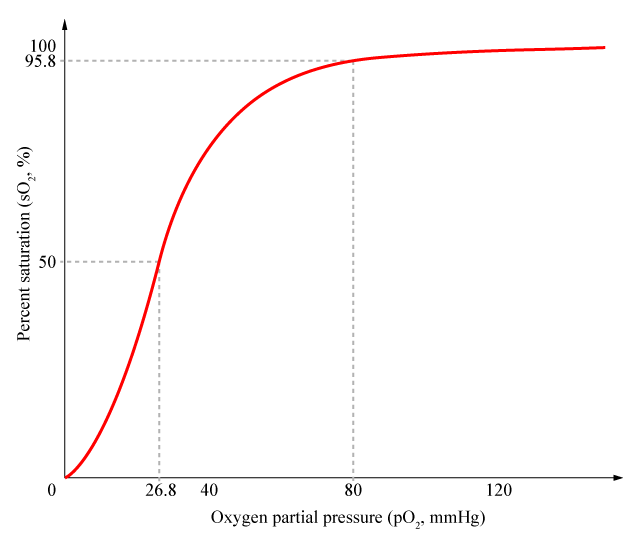
Cooperativity is a common phenomenon in biological systems involving multiple ligands binding to enzymes or receptors. The change in affinity of a binding site for a ligand upon a ligand-binding leads to either positive cooperativity in which affinity is increased and subsequent binding become more likely, or negative cooperativity in which affinity is decreased to hinder future binding. One of the most famous examples of cooperative binding is the binding of oxygen to hemoglobin, the oxygen-transporting protein in red blood cells. When an oxygen molecule bind to hemoglobin, its affinity for oxygen increases greatly, and when three oxygen molecules bind (3-oxy-hemoglobin), its affinity for the fourth one is nearly three-hundred times higher than deoxy-hemoglobin’s affinity for oxygen. This cooperative property leads to the sigmoidal shaped curve shown below. | Cooperativity is a common phenomenon in biological systems involving multiple ligands binding to enzymes or receptors. The change in affinity of a binding site for a ligand upon a ligand-binding leads to either positive cooperativity in which affinity is increased and subsequent binding become more likely, or negative cooperativity in which affinity is decreased to hinder future binding. One of the most famous examples of cooperative binding is the binding of oxygen to hemoglobin, the oxygen-transporting protein in red blood cells. When an oxygen molecule bind to hemoglobin, its affinity for oxygen increases greatly, and when three oxygen molecules bind (3-oxy-hemoglobin), its affinity for the fourth one is nearly three-hundred times higher than deoxy-hemoglobin’s affinity for oxygen. This cooperative property leads to the sigmoidal shaped curve shown below. | ||
| + | |||
| + | [[File:Cooperativity.png|400px|center]] | ||
| + | <div align="center"> Figure 1. Cooperativity of Hemoglobin</div> <br><br> | ||
<br><br><br> | <br><br><br> | ||
Revision as of 23:34, 17 September 2013
Mathematical Modeling of Bistable Toggle Switch
Cooperativity
Cooperativity is a common phenomenon in biological systems involving multiple ligands binding to enzymes or receptors. The change in affinity of a binding site for a ligand upon a ligand-binding leads to either positive cooperativity in which affinity is increased and subsequent binding become more likely, or negative cooperativity in which affinity is decreased to hinder future binding. One of the most famous examples of cooperative binding is the binding of oxygen to hemoglobin, the oxygen-transporting protein in red blood cells. When an oxygen molecule bind to hemoglobin, its affinity for oxygen increases greatly, and when three oxygen molecules bind (3-oxy-hemoglobin), its affinity for the fourth one is nearly three-hundred times higher than deoxy-hemoglobin’s affinity for oxygen. This cooperative property leads to the sigmoidal shaped curve shown below.
Hill Equation
 "
"