Team:TU-Eindhoven/MRIProcessing
From 2013.igem.org
Pascalaldo (Talk | contribs) (→The Principle) |
Pascalaldo (Talk | contribs) (→The Principle) |
||
| Line 14: | Line 14: | ||
<html>$$\begin{aligned} | <html>$$\begin{aligned} | ||
MTR_{asym} &= {{S_{w}^{-\Delta\omega_{sw}} - S_{w}^{-\Delta\omega_{sw}}}\over{S_0}} \\ | MTR_{asym} &= {{S_{w}^{-\Delta\omega_{sw}} - S_{w}^{-\Delta\omega_{sw}}}\over{S_0}} \\ | ||
| - | PTR &= {{2 \cdot [H_{2}O]}\over{[polypeptide]}} | + | PTR &= {{2 \cdot [H_{2}O]}\over{[polypeptide]}} \cdot MTR_{asym} |
\end{aligned} | \end{aligned} | ||
$$ | $$ | ||
Revision as of 14:39, 3 October 2013



Contents |
MRI Data Processing
Until recently, the most common way of generating MRI contrast was by using heavy metal based contrast agents. Examples of these substances are the commercial Gadolinium(III) based Magnevist and the Iron Oxide based Resovist. A very obvious downside of using heavy metals is the toxicity of the agents in the human body. Fortunately, a new type of MRI has been developed, which allows for the use of organic compounds as contrast agent.
The Principle
This new type of MRI goes by the name Chemical Exchange Saturation Transfer MRI or CEST MRI. The principle behind this technique is based on compounds that contain pools of exchangeable protons that can be selectively saturated using radiofrequency irradiation. Upon proton exchange with bulk water, these compounds can be indirectly visualized by measuring the bulk water. The amino acids lysine, arginine, threonine and serine contain those exchangeable protons and polypeptides containing those amino acids in abundance are therefore potential contrast agents. An other advantage of CEST MRI is that different kind of protons (e.g. amide and hydroxide protons) can be distinguished, which allows for 'multi-color' imaging using multiple contrast agents simultaneously.
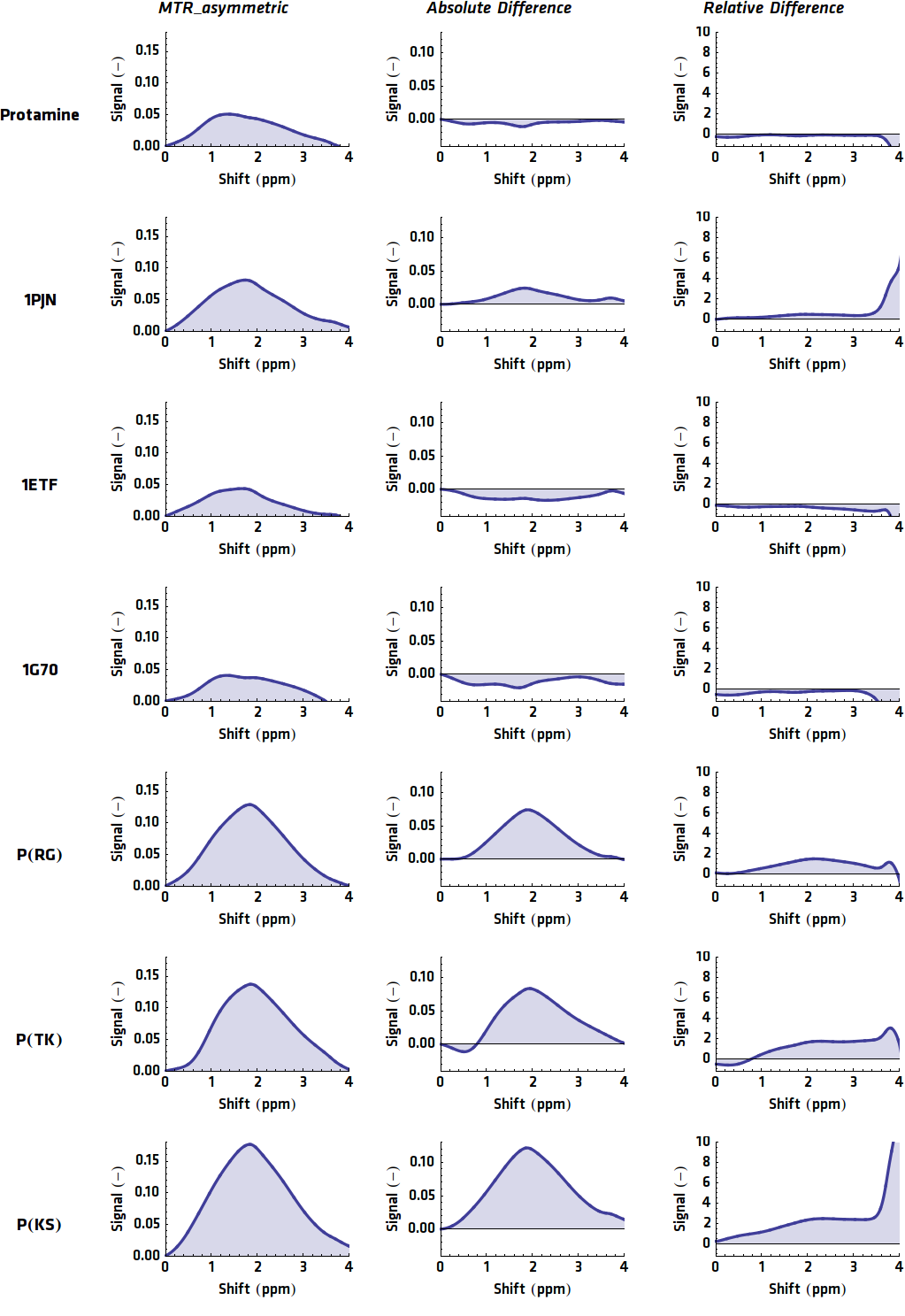
To detect these protons in bulk water some processing steps are required. First of all, the imaging should be taken at different frequencies of the saturation pulse. This way the signal at different chemical shifts can be measured. When bulk water is set to 0 ppm, the signal plot will be roughly symmetric around zero, since saturation pulses will excite the bulk water in a similar way when the absolute shift is equal. This also implies that when the signals with a negative shift from the bulk water are compared with the signals with a positive shift, asymmetry can be detected. The CEST effect is exactly what causes asymmetry in the signal plot, because the chemical shift of the CEST compounds is different from the chemical shift of water. Therefore, when the absolute shift from bulk water of two saturation pulses is equal, this does not mean the absolute shift from the CEST compound is equal and the CEST protons will in one case get barely saturated (depending on its shift from bulk water of course).
Putting this principle into practice yields the processing steps. First of all, the asymmetric comparison should be conducted:
Results
References
 "
"



