Team:TU-Delft/Peptides
From 2013.igem.org
| Line 89: | Line 89: | ||
<h4>Results</h4> | <h4>Results</h4> | ||
| + | |||
| + | |||
| + | <p align="justify"> | ||
| + | The presence of plasmid with gene inserts encoding the SUMO-peptide ( | ||
| + | <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1022101" target="blank">BBa_K1022101</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1022102" target="blank">BBa_K1022102</a>,<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1022103" target="blank">BBa_K1022103</a>) was confirmed by a colony pcr. The expected size of the insert with the T7 promoter was approximately around 400 bp which was clearly evident from the agarose gel picture Fig (agarose). An Eurogentec Smartladder MW-1700-10 (<a href="https://secure.eurogentec.com/uploads/TDS-MW-1700-10.pdf">https://secure.eurogentec.com/uploads/TDS-MW-1700-10.pdf</a>) was used to identify the size of the fragments. | ||
| + | </p> | ||
<h4>Discussion</h4> | <h4>Discussion</h4> | ||
Revision as of 18:43, 3 October 2013

Content
Peptide Production
SUMO-Peptide Production
SUMO cleavage by Ulp1
Peptide production
The era of antibiotics are now outdated as the microorganisms started gaining new resistance mechanism to evade the effects of antibiotics. Nowadays more and more researches are focused towards new antimicrobial that can be more effective against these pathogens.
The antimicrobial peptides (AMP), natural or de-novo are becoming more popular due to their potential to kill or restrict the growth of microorganisms. They form a part of chemical defense against predators. All organisms, including higher order eukaryotes have some antimicrobial peptides that acts against specific strains of microorganisms.They are usually charged and interact with the opposite charges present on the membrane thus boring a hole through the membrane subsequently lysing the cell [1].Unlike, antibiotics they do not interfere in the genetic, transcriptional or translational machinery of the cells.
We chose 3 AMP that are produced in the skins of toads and frogs. They are magainin (GIGKFLHSAKKFGKAFVGQIMNS) from Xenopus laevis , signiferin (IIGHLIKTALGMLGL) from Crinia signifera and maximin-H5 (ILGPVLGLVSDTLDDVLGIL) from Bombina maxima. The reasons for choosing these AMP are because of their target specific nature, charge and length. They do not affect humans, which makes them a suitable candidate as pharmaceuticals [2].
The recombinant production of these peptides in E.coli would help large scale production of these peptides and administer them as antimicrobials. But, the major hurdle in producing them in vivo is the formation of inclusion bodies as they are charged. This could be overcome by making protein fusion with SUMO (Small Ubiquitin like Modifier), MBP (Maltose Binding Protein), Trx (Thioredoxin) etc.
In this project, a SUMO-peptide fusion was opted [3] as a suitable expression system as they make the fusion proteins more soluble. The Ulp-1 cleaving the fusion is a special protease that can cleave of the fusion without leaving any scar. This protease is also expressed and controlled by a negative transcriptional cascade. As the fusion is produced it triggers a cascade that ultimately leads to the expression of Ulp-1 and facilitates in vivo cleavage. This fusion increases the solubility of the peptide and reduces inclusion bodies formation.(Figure 1).

Figure 1: Schematic diagram of the peptide production
SUMO-Peptide Production
Aim: Increasing solubility of peptide with Sumo Fusion.
Description
The peptide by itself is not soluble in the cytoplasm but making a fusion of peptide with Small Ubiquitin like Modifiers (SUMO) will increase the solubility of the peptide, thus increasing the cytoplasmic fraction of the peptide.
A gene was constructed in such a way that the SUMO-peptide production was driven by the strong T7 phage promoter. This gene containing plasmid was harboured in a BL21(DE3) strain that has lac promoter driven T7 polymerase. Upon induction by IPTG the SUMO peptide fusion is produced as a soluble protein fraction.
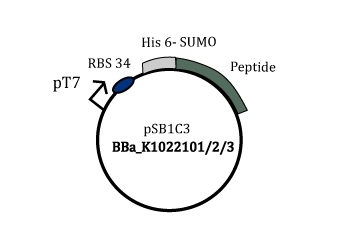
Figure 2: Part BBa_K1022101/2/3
Results
The presence of plasmid with gene inserts encoding the SUMO-peptide ( BBa_K1022101, BBa_K1022102,BBa_K1022103) was confirmed by a colony pcr. The expected size of the insert with the T7 promoter was approximately around 400 bp which was clearly evident from the agarose gel picture Fig (agarose). An Eurogentec Smartladder MW-1700-10 (https://secure.eurogentec.com/uploads/TDS-MW-1700-10.pdf) was used to identify the size of the fragments.
Discussion
SUMO cleavage by Ulp1
Cleavage of SUMO from Peptide by Ulp-1 protease.
The SUMO-peptide helps in increasing the soluble fraction of peptide but peptides are not biologically active in a fusion, they have to be cleaved from the fusion to get a active peptide fraction. This was achieved by in vivo production on SUMO specific Ulp-1 proteases.
A gene was constructed in such a way that the SUMO-peptide production was driven by the strong T7 phage promoter and the Ulp-1 production was driven by arabinose inducible promoter pBad. The plasmid was transformed into an BL21(DE3) pLysS strain. This construct was designed to check whether in vivo cleavage is possible. The main idea of the experiment is to first produce large amount of soluble fraction of SUMO-peptides and the produce the Ulp protease to cleave the sufficiently produced fusion peptides.

Figure 3: Part BBa_K1022104/5/6
Results
Discussion
References
- Michael R. Yeaman & Nannette Y. Yount,..(2003) 'Mechanisms of Antimicrobial Peptide Action and Resistance' , Pharmacological Reviews,vol 55,p:27-55
- Wang, Z. and Wang, G.* (2004) APD: the Antimicrobial Peptide Database. Nucleic Acids Research 32, D590-D592
- Panavas T, Sanders C, Butt TR.. (2009). SUMO fusion technology for enhanced protein production in prokaryotic and eukaryotic expression systems. Methods in Molecular Biology. 10 (8), p4-20.
 "
"
