Team:TU-Delft/FinalApplication
From 2013.igem.org
| Line 137: | Line 137: | ||
<center> | <center> | ||
| - | + | ||
Table 1: Cellulose Nitrate Membrane, 0.1μm pore size, peptides testing | Table 1: Cellulose Nitrate Membrane, 0.1μm pore size, peptides testing | ||
| - | + | ||
</center> | </center> | ||
| Line 155: | Line 155: | ||
</tr> | </tr> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| Line 289: | Line 274: | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| Line 407: | Line 379: | ||
<br> | <br> | ||
| - | Table | + | Table 3: Cellulose Nitrate Membrane, 0.1μm pore size, AIPs testing |
</b> | </b> | ||
</center> | </center> | ||
| Line 426: | Line 398: | ||
</td> | </td> | ||
<td > | <td > | ||
| - | + | 0.04 | |
</td> | </td> | ||
<td> | <td> | ||
| - | + | 0.02 | |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 452: | Line 424: | ||
</td> | </td> | ||
<td> | <td> | ||
| - | 0. | + | 0.23 |
</td> | </td> | ||
| - | <td>0. | + | <td>0.14</td> |
| Line 465: | Line 437: | ||
</td> | </td> | ||
<td > | <td > | ||
| - | 0. | + | 0.20 |
</td> | </td> | ||
<td> | <td> | ||
| - | 0. | + | 0.12 |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 478: | Line 450: | ||
</td> | </td> | ||
<td > | <td > | ||
| - | 0. | + | 0.25 |
</td> | </td> | ||
<td> | <td> | ||
| - | 0. | + | 0.16 |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 491: | Line 463: | ||
</td> | </td> | ||
<td > | <td > | ||
| - | 0. | + | 0.28 |
</td> | </td> | ||
<td> | <td> | ||
| - | 0. | + | 0.18 |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 504: | Line 476: | ||
</td> | </td> | ||
<td > | <td > | ||
| - | 0. | + | 0.32 |
</td> | </td> | ||
<td> | <td> | ||
| - | 0. | + | 0.2 |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 517: | Line 489: | ||
</td> | </td> | ||
<td > | <td > | ||
| - | 0. | + | 0.37 |
</td> | </td> | ||
<td> | <td> | ||
| - | 0. | + | 0.23 |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 530: | Line 502: | ||
</td> | </td> | ||
<td > | <td > | ||
| - | 0. | + | 0.41 |
</td> | </td> | ||
<td> | <td> | ||
| - | 0. | + | 0.26 |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 544: | Line 516: | ||
</td> | </td> | ||
<td > | <td > | ||
| - | 0. | + | 0.45 |
</td> | </td> | ||
<td> | <td> | ||
| - | 0. | + | 0.28 |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 557: | Line 529: | ||
</td> | </td> | ||
<td > | <td > | ||
| - | 0. | + | 0.48 |
</td> | </td> | ||
<td> | <td> | ||
| - | 0. | + | 0.30 |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 571: | Line 543: | ||
</td> | </td> | ||
<td > | <td > | ||
| - | 0. | + | 0.53 |
</td> | </td> | ||
<td> | <td> | ||
| - | 0. | + | 0.34 |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 584: | Line 556: | ||
</td> | </td> | ||
<td > | <td > | ||
| - | 0. | + | 0.81 |
</td> | </td> | ||
<td> | <td> | ||
| - | 0. | + | 0.52 |
</td> | </td> | ||
</tr> | </tr> | ||
</table> | </table> | ||
</div> | </div> | ||
| + | <br><br> | ||
| - | + | Table 4: PTFE Membrane, 0.1μm pore size, AIPs testing | |
| - | Table : PTFE Membrane, 0.1μm pore size, AIPs testing | + | |
</b> | </b> | ||
</center> | </center> | ||
Revision as of 11:15, 4 October 2013

BandAid Application
The Band Aid is related to the final application of our project. Specifically, the idea is to use a band aid in which E. coli will be present. After placing the band aid on the wound, S.aureus will be detected, the peptide will, after production, diffuse through the band aid targeting the infection. Diffusion of the AIP and the AMP have been shown in the membrane experiment.

Figure 1: Band aid
Experiments
Aim: To check if it is feasible for both the peptides and the AIPs to pass through the selected membranes (Cellulose Nitrate Membrane and PTFE Membrane).
Procedure:- Dissolve the peptides. Signiferin was dissolved in water, in order to dissolve maximim 1M of ammonium bicarbonate was added in order to gain an end concentration of 5mM.
- The peptide solutions were measured, using the nanodrop, and we determined the absorbance to be 0.51 and 0.74 respectively. Using absorbance A= εcL=εc when L = 1cm [1], [2] the concentration was measured to be 0.34μM for Maximim and 0.49μM for Signiferin.
- In order to make diffusion measurable, we folded the membrane like a filter paper and placed it in the peptide solution.
- The diffusion was measured from the upper part of the membrane in different time points.
- The peptides cannot pass through the PTFE membrane which is hydrophobic(Table 2)
- Both the peptides are successfully passing through the Cellulose Nitrate Membrane(Table 1)
- Signiferin passes through the Cellulose Nitrate Membrane in a higher concentration than Maximim (Figure 2). The slow diffusion of Maximim is probably based on the fact that Maximim has a negative charge of -3 whereas Signiferin a positive charged of 2.[3]
-
Pierce Biotechnology: Extinction Coefficients, A guide to understanding extinction coefficients, with emphasis on spectrophotometric determination of protein concentration.[Online]. Available From: http://classes.soe.ucsc.edu/bme220l/Spring11/Reading/Extinction-coefficients.pdf
C. N. Pace, F. Vajdos, L. Fee, G. Grimsley, and T. Gray,"How to measure and predict the molar absorption coefficient of a protein.", Protein Sci. 1995 November; 4(11): 2411–2423.
Peptide Calculator[Online]. Available From https://www.genscript.com/ssl-bin/site2/peptide_calculation.cgi
| minutes | Absorbance(Maximim) | Absorbance(Signiferin) | Concentration in mM Maximim | Concentration in mM Signiferin |
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0.03 | 0 | 0.02 | 0 |
| 3 | 0.01 | 0.08 | 0.006 | 0.05 |
| 20 | 0.02 | _ | 0.013 | - |
| 60 | 0.16 | 0.27 | 0.1 | 0.18 |
| 80 | 0.2 | 0.44 | 0.13 | 0.29 |
Table 2: PTFE Membrane, 0.1μm pore size, peptide testing
| minutes | Absorbance(Maximim) | Absorbance(Signiferin) | Concentration in mM Maximim | Concentration in mM Signiferin |
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 |
| 20 | 0 | 0 | 0 | 0 |
| 60 | 0 | 0 | 0 | 0 |
| 80 | 0 | 0 | 0 | 0 |
Table 3: Cellulose Nitrate Membrane, 0.1μm pore size, AIPs testing
| minutes | Absorbance(AIP) | Concentration in mM AIP |
| 1 | 0.04 | 0.02 |
| 5 | 0.02 | 0.01 |
| 10 | 0.23 | 0.14 |
| 15 | 0.20 | 0.12 |
| 30 | 0.25 | 0.16 |
| 60 | 0.28 | 0.18 |
| 65 | 0.32 | 0.2 |
| 75 | 0.37 | 0.23 |
| 105 | 0.41 | 0.26 |
| 135 | 0.45 | 0.28 |
| 175 | 0.48 | 0.30 |
| 200 | 0.53 | 0.34 |
| 250 | 0.81 | 0.52 |
Table 4: PTFE Membrane, 0.1μm pore size, AIPs testing
| minutes | Absorbance(AIP) | Concentration in mM AIP |
| 1 | -0.01 | -0.02 |
| 5 | 0.02 | 0.01 |
| 10 | 0.04 | 0.02 |
| 15 | 0.02 | 0.01 |
| 30 | 0.06 | 0.03 |
| 60 | 0.02 | 0.01 |
| 65 | 0.04 | 0.02 |
| 75 | 0.04 | 0.02 |
| 105 | 0.04 | 0.02 |
| 135 | 0.05 | 0.03 |
| 175 | 0.03 | 0.01 |
| 200 | 0.08 | 0.05 |
| 250 | 0.06 | 0.03 |
Graphs
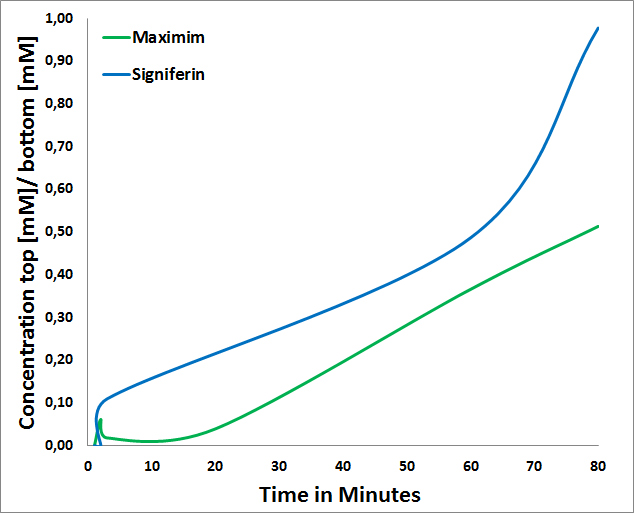
Figure 2:Maximim and Signiferin ratio passing through membrane
Conclusions
The conclusions derived by the experiments are listed below:
All these facts make the cellulose nitrate membrane to be a proper membrane for our application in contrast to the PTFE membrane which does not allow the peptides to pass through due to its hydrophobic nature. The time that the peptide needs to pass through the membrane is reasonable for a real application. It is possible to reduce the time needed by increasing the pore size of the membrane. However, it was decided not to do this in order to be impossible for the E.coli to escape from the membrane and infect the patient.
 "
"
