Team:Paris Saclay/Notebook/July/16
From 2013.igem.org
(Difference between revisions)
(→2 - Electrophoresis of the digestion of PSB3K3 by EcoRI/PstI) |
(→1 - Electrophoresis of the digestion of BBa_K1155001, BBa_K1155002, BphR2 in pSB1C3) |
||
| Line 109: | Line 109: | ||
* Gel : 1.5% | * Gel : 1.5% | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
{| | {| | ||
| Line 118: | Line 114: | ||
We didn't obtain fragments at the right size. We will do the ligation again. | We didn't obtain fragments at the right size. We will do the ligation again. | ||
|} | |} | ||
| - | |||
| - | |||
{| | {| | ||
| Line 129: | Line 123: | ||
* Gel : 1.5% | * Gel : 1.5% | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
{| | {| | ||
Latest revision as of 00:02, 5 October 2013
Notebook : July 16
Lab work
A - Aerobic/Anaerobic regulation system
Objective : obtaining BBa_K1155003, BBa_K1155007
1 - Extraction of BBa_B0015, BBa_B0017, BBa_I732019 from DH5α
Zhou
Protocol : High copy plamid extraction
2 - Digestion of BBa_B0015, BBa_B0017, BBa_I732019 by EcoRI/PstI
Anaïs
- DNA : 5µL
- Buffer FD : 2µL
- EcoRI FD : 1µL
- PstI FD : 1µL
- H2O : 21µL
We let our digestion 1h30 at 37°C.
Objective : obtaining biobricks in PSB3K3
1 - Digestion of pSB3K3 by EcoRI/PstI
Anaïs
Used quantities :
- DNA : 5µL
- Buffer FD : 2µL
- EcoRI FD : 1µL
- PstI FD : 1µL
- H2O : 11µL
We let our digestion 1h30 at 37°C.p
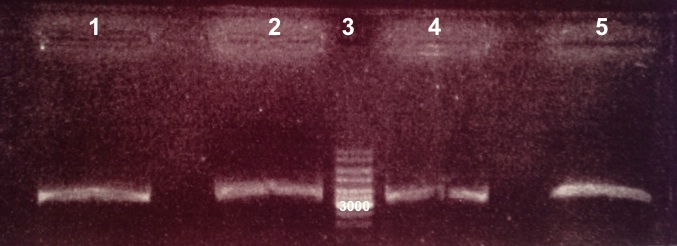
2 - Electrophoresis of the digestion of pSB3K3 by EcoRI/PstI
Sheng
Expected sizes :
- pSB3K3 : 2750bp
|
We obtain fragments at the right size. |
3 - Gel purification of electrophoresis of the digestion of pSB3K3 by EcoRI/PstI
Abdou
Protocol : [http://www.mn-net.com/tabid/1452/default.aspx Gel purification ]
B - PCB sensor system
Objective : obtaining BBa_K1155001, BBa_K1155002, BphR2 in pSB1C3
1 - Electrophoresis of the digestion of BBa_K1155001, BBa_K1155002, BphR2 in pSB1C3
Anaïs, Zhou
- BBa_K1155001 :

|
|
Expected size :
- BBa_K1155001 digested by EcoRI/PstI : 2037bp + 333bp
- BBa_K1155001 digested by SacII : 2370bp
|
We obtain fragment at the right size. We will amplify BBa_K1155001. |
- BBa_K1155002 :
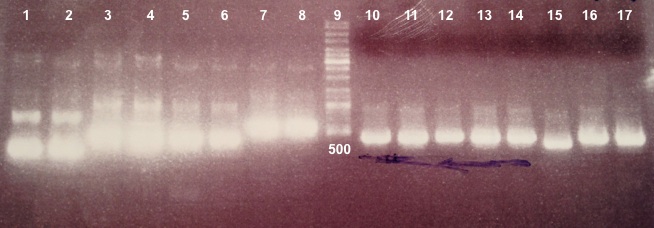
|
|
|
We didn't obtain fragments at the right size. We will do the ligation again. |
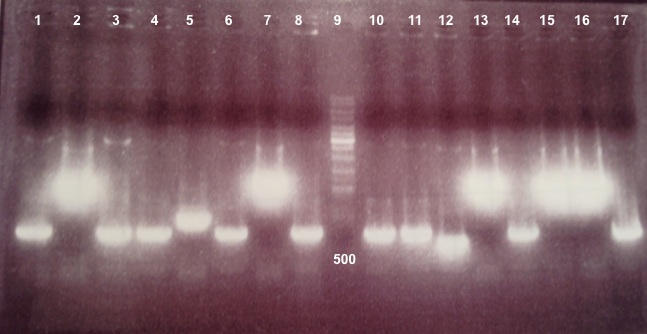
|
|
|
We didn't obtain fragments at the right size. After reading the sequence again, we find an digestion site in the middle of our biobrick. We will do a Gibson assembly to modify this site. |
| Previous day | Back to calendar | Next day |
 "
"