Team:Evry/Modelmeta1
From 2013.igem.org
| Line 45: | Line 45: | ||
<h2>Materials and methods</h2> | <h2>Materials and methods</h2> | ||
<p> | <p> | ||
| + | <u><b>From Iron to FBS:</b></u><br/> | ||
The iron-FUR complex is simply formed that way:<br/> | The iron-FUR complex is simply formed that way:<br/> | ||
<img src="https://static.igem.org/mediawiki/2013/7/72/Reg1.png"/><br/> | <img src="https://static.igem.org/mediawiki/2013/7/72/Reg1.png"/><br/> | ||
| Line 60: | Line 61: | ||
In this model, we only track the free Fe-FUR complex and not those attached to a FUR Binding Site. <i>FBS</i> is the number of inhibited Fur Binding Sites.<br/> | In this model, we only track the free Fe-FUR complex and not those attached to a FUR Binding Site. <i>FBS</i> is the number of inhibited Fur Binding Sites.<br/> | ||
<img src="https://static.igem.org/mediawiki/2013/6/6d/Regfefur.png"/> | <img src="https://static.igem.org/mediawiki/2013/6/6d/Regfefur.png"/> | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | <u><b>GFP expression:</b></u><br/> | ||
| + | To simulate the inhibition phenomenon, we chose to use our <a href="https://2013.igem.org/Team:Evry/LogisticFunctions">logistic function</a> under its differential form. Since it is the Fe-FUR that represses it, the LacI can be expressed as a logistic fuction of the Fe-FUR:<br/> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/4/49/Dlaci.png"/><br/> | ||
| + | Ki1 is a non-dimensional parameter that repesents the inhibition power, and Kf is the fixation rate of the Fe-FUR on the FBS. Finally, Nbrpla1 is the number of pasmids containing the LacI.<br/> | ||
| + | In the same way, LacO is modeled with a logistic funtion. LacO is repressed by LacI:<br/> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/f/f2/Dlaco.png"/><br/> | ||
| + | Ki2 is the inhibition power and Nbrpla2 is the number of plasmimds containing LacO.<br/> | ||
| + | LacI and LacO are both ruled by a normal logistic function. If we were to track the number of expressed LacI/LacO, we would be using two inverted logistic fuctions to model a double inverter. The thing is, since <i>LacI</i> represents the number of <b>repressed</b> genes and <i>LacO</i> the number of <b>expressed</b> genes, the double inverter is still there, but the calculations are easier.<br/> | ||
| + | </p> | ||
| + | |||
| + | <p> | ||
| + | <u><b>GFP Production:</b></u><br/> | ||
| + | The [mRNA] and [Enz] equations are alike. The prodction rates are Kr for the mRNA and Kp for the enzymmes, and both variables have a negative degadation term:<br/> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/3/31/Dmrnadenz.png"/> | ||
</p> | </p> | ||
Revision as of 15:28, 27 October 2013
Sensor Model
Introduction
In order to determine our Iron Coli's enterobactin production rate, we first have to know how much time our bacteria will take to sense the ambient iron. So, this first part of the Enterobactin production model focuses on the synthetic sensing system our team implemented in the bacteria.
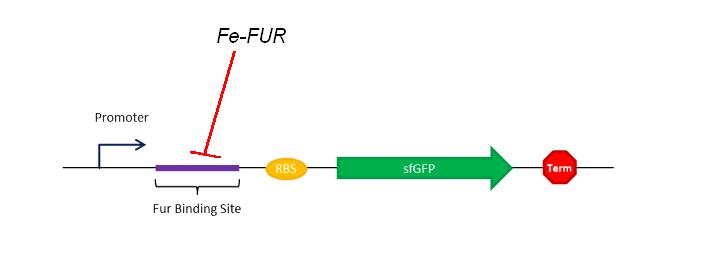
Observations
As shown on the Figure 1, our sensing system relies on a FUR Binding Site (FBS). In order to easily model the sensing delay and analyse its results, the first construction is composed of a GFP placed right after the FBS.
Goals
Our goal in this part of the model is to create a generic FBS-related sensing model so that:
- We can determine the iron-sensing delay of our bacteria
- The model can can be reused by other iron related projects
Materials and methods
From Iron to FBS:
The iron-FUR complex is simply formed that way:

We reduced this equation to:

Which is not annoying, since we just have to divide our [FeFur] by two to get the real complex concentration.
We can easily write down both the formation (v) and the dissociation (v') speeds:

We chose to model the iron input in the bacteria using a linear function of the external iron concentration Ferext, the factor p being the cell-wall permeability for iron.
The FUR on the other hand is produced by the bacteria. Its evolution can also be considered as linear, using a mean production rate of Fur0.


In this model, we only track the free Fe-FUR complex and not those attached to a FUR Binding Site. FBS is the number of inhibited Fur Binding Sites.

GFP expression:
To simulate the inhibition phenomenon, we chose to use our logistic function under its differential form. Since it is the Fe-FUR that represses it, the LacI can be expressed as a logistic fuction of the Fe-FUR:

Ki1 is a non-dimensional parameter that repesents the inhibition power, and Kf is the fixation rate of the Fe-FUR on the FBS. Finally, Nbrpla1 is the number of pasmids containing the LacI.
In the same way, LacO is modeled with a logistic funtion. LacO is repressed by LacI:

Ki2 is the inhibition power and Nbrpla2 is the number of plasmimds containing LacO.
LacI and LacO are both ruled by a normal logistic function. If we were to track the number of expressed LacI/LacO, we would be using two inverted logistic fuctions to model a double inverter. The thing is, since LacI represents the number of repressed genes and LacO the number of expressed genes, the double inverter is still there, but the calculations are easier.
GFP Production:
The [mRNA] and [Enz] equations are alike. The prodction rates are Kr for the mRNA and Kp for the enzymmes, and both variables have a negative degadation term:

Results
Conclusion
Models and scripts
References:
 "
"