Team:Paris Saclay/Notebook/August/14
From 2013.igem.org
| Line 163: | Line 163: | ||
Nanodrop : | Nanodrop : | ||
| - | * RSB-LacZ-Term : | + | * RSB-LacZ-Term : 59.6ng/µL |
* RBS-AmilCP-Term : 28.7ng/µL | * RBS-AmilCP-Term : 28.7ng/µL | ||
Revision as of 18:52, 20 September 2013
Notebook : August 14
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize Bba_K1155000, Bba_K1155004, Bba_K1155005, Bba_K1155006
1 - Gel purification of the digestion of Bba_K1155004, Bba_K1155005, Bba_K1155006 by SpeI
XiaoJing
Protocol : Gel purification
Nanodrop :
- NarG : 10.5ng/µL
- NarK : 16.8ng/µL
- NirB : 24.3
|
We lost our plasmids. We will do the digestion again. |
2 - Extraction of Bba_K1155000, Bba_K1155004, Bba_K1155005, Bba_K1155006 from DH5α
XiaoJing
Protocol : Hight copy plamid extraction
Nanodrop :
- NarK: 89.8ng/µL
- NarG : 80.7ng/µL
- Nir B :65.8ng/µL
- Pfnr : 227ng/µL
|
The extraction was good. We will digest Bba_K1155000, Bba_K1155004, Bba_K1155005, Bba_K1155006. |
3 - Digestion of Bba_K1155004,Bba_K1155005, Bba_K1155006 by EcoRI/SpeI
Nadia
- Buffer FD : 2µL
- H2O : 6µL
- DNA : 10µL
- SpeI FD : 1µL
- EcoRI FD : 1µL
We let the digestion at 37°C during 15 minutes.
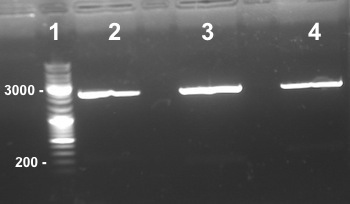
4 - Electrophoresis to check the digestion of Bba_K1155004,Bba_K1155005, Bba_K1155006 by EcoRI/SpeI
Anaïs, Nadia
Expected sizes :
- PSB1C3 : 2070kb
- NarK, NarG, NirB : 200kb
|
We obtained NarK, NarG, NirB fragments at the right size but in very few quantity. We do it again but this time we will use more quantity of enzymes. |
5 - Digestion of Bba_K1155000, Bba_K1155004,Bba_K1155005, Bba_K1155006 by SpeI and EcoRI/SpeI
Anaïs, Nadia
- Digestion by SpeI :
- Buffer : 2µL
- SpeI : 2µL
- ADN : 15µL
- H20 : 1µL
- Digestion by EcoRI and SpeI :
- Buffer : 3µL
- SpeI : 2µL
- EcoRI : 2µL
- ADN : 20µL
- H20 : 3µL
We let digestions at 37°C during 10 minutes ??????
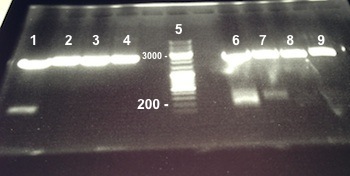
6 - Electrophoresis to check the digestion of Bba_K1155000, Bba_K1155004,Bba_K1155005, Bba_K1155006 by SpeI and EcoRI/SpeI
Anaïs, Nadia
Expected sizes :
- Pfnr in PSB1C3 : ...
- Nar K in PSB1C3, NarG in PSB1C3, NirB in PSB1C3 : ...
- PSB1C3 : 2070kb
- NarK, NarG, NirB : 200kb
- Pfnr : ...
|
Well 1 : we have done one digestion so we have to obtain one fragment, we have two stripes, the digestion of Bba_K1155000 by SpeI wasn't good. Well 2, 3, 4, 6, 7 : we obtain NarG, NarK and NirB in PSB1C3 and Pfnr, NarK fragments at the right size, we can purify it. Well 8, 9 : we have done two digestions so we have to obtain two fragments, we have only one stripe, the digestion of Bba_K1155005 and Bba_K1155004 by EcoRI/SpeI wasn't good. |
7 - Gel purification of the digestion of Bba_K1155000 and Bba_K1155006 by EcoRI/SpeI and Bba_K1155004, Bba_K1155005 and Bba_K1155006 by SpeI
Anaïs, Nadia
Protocol : Gel purification
| [[]] |
|
Nanodrop :
- Pfnr : 13.7ng/µL
- NarK : 27.9ng/µL
- NarK in PSB1C3 : 87.1ng/µL
- NarG in PSB1C3 : 39.2ng/µL
- NirB in PSB1C3 : 37.7ng/µL
|
CONCLUSION pour Pfnr faible quantité d'où on recommence plus tard ?,????? |
8 - Gel purification of the digestion of Bba_K1155003 and Bba_K1155007 by XBaI/PstI
Nadia
| [[]] |
|
Nanodrop :
- RSB-LacZ-Term : 59.6ng/µL
- RBS-AmilCP-Term : 28.7ng/µL
|
WE obtain few quantities of plasmid that's why we will make an Ethanol precipitation to concentrated it. |
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining FNR and BphR2 proteins
1 - Gibson assembly
Used quantities :
- RBS-BphR2 :
- PSB1C3 : 3µL
- BphR2 Part I : 1µL
- BphR2 Part II : 1µL
- Gibson mix : 15µL ???????????????
- FNR :
- PSB1C3 : 3µL
- FNR Part I : 1µL
- FNR Part II : 1µL
- Gisbon mix : 15µL ???????????????
- RBS-FNR :
- PSB1C3 : 3µL
- RBS-FNR Part I : 1µL
- FNR Part II : 1µL
- Gibson mix : 15µL ????????????
We let these mix at 50°C during 1h. Then we the these mix at 4°C during the week end.
2 - Electrophoresis of PCR products : BphR2 Part I
Damir
| [[]] |
|
| [[]] |
|
Expected sizes :
- BphR2 Part I : 178kb
|
On the first gel, all deposits disappear so we did the electrophoresis again. On the second gel, we didn't obtain stripes at the good size. We do the PCR again using new quantities and a new PCR program. |
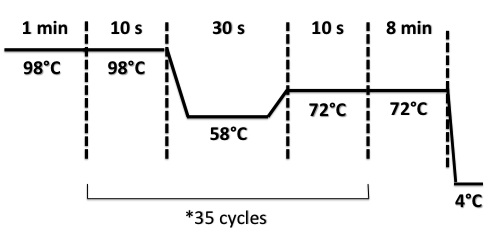
3 - PCR of BphR2 Part I
Damir
Used quantities :
- Oligo 54F : 2µL
- Oligo 55R : 2µL
- DNA : 1µL
- Buffer Phusion : 10µL
- dNTP : 1µL
- Phusion : 1µL
- DMS9 : 2µL ??????????????????????????????????
- H2O : 31µL
PCR program :
 "
"