|
|
| Line 1: |
Line 1: |
| - | ='''Notebook : August 30'''=
| |
| - |
| |
| - | =='''summary'''==
| |
| - | * check the size by Gel electrophoresis for PCR clonies on Gibson assembly and ligation promoter fnr(repressor) plus RBS_AmilCP+Term_PSB1C3 .
| |
| - | * Do ligation for For promoter fnr(repressor)+ RBS_LacZ+Term_PSB1C3 , promoter fnr(activator)nirK + RBS_LacZ+Term_PSB3K3 and promoter fnr(repressor) + RBS_LacZ+Term_PSB3K3 and Transformation and
| |
| - | incubation.
| |
| - |
| |
| - |
| |
| - | <br>
| |
| - |
| |
| - | =='''lab work'''==
| |
| - |
| |
| - | <br>
| |
| - | *'''A.aero/anaerobic regulation system'''<br>
| |
| - |
| |
| - |
| |
| - | ===='''1 -Gel electrophoresis of PCR clonies on Gibson assembly .'''====
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - | {|
| |
| - | | style="width:350px;border:1px solid black;" | [[]]
| |
| - | | style="width:350px;border:1px solid black;vertical-align:top;" |
| |
| - | *Well 1 : 6µL DNA Ladder
| |
| - | *Well AA 1-8 : Clonies from Gibson FNR_part1, FNR part1 and plasmid PSB1C3
| |
| - | *Well AB 1-8 : Clonies from Gibson FNR_part1, FNR part1 and plasmid PSB1C3 Concentrate3
| |
| - | *Well AC 1-8 :Clonies from Gibson RBS_BphR2_part1, BphR2_part2 and plasmid PSB1C3
| |
| - | *Well AD 1-8 :Clonies from Gibson RBS_FNR part1, FNR_part2 and plasmid PSB1C3
| |
| - | *Well AE 1-8 :Clonies from Gibson RBS_FNR part1, FNR_part2 and plasmid PSB1C3 Concentrate
| |
| - | *Well AF 1-8 :Clonies from Gibson RBS_BphR2_part1, BphR2_part2 and plasmid PSB1C3 Concentrate
| |
| - |
| |
| - | *Gel : 1.0%
| |
| - | |}
| |
| - |
| |
| - | ===='''2 -Gel electrophoresis of ligation promoter fnr(repressor) plus RBS_AmilCP+Term_PSB1C3 .'''====
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - | {|
| |
| - | | style="width:350px;border:1px solid black;" | [[]]
| |
| - | | style="width:350px;border:1px solid black;vertical-align:top;" |
| |
| - | *Well 0: 6µL DNA Ladder
| |
| - | *Well 1: clone1 of ligation promoter fnr(repressor) plus RBS_AmilCP+Term_PSB1C3
| |
| - | *Well 2: clone2 of ligation promoter fnr(repressor) plus RBS_AmilCP+Term_PSB1C3
| |
| - | *Well 3: clone3 of ligation promoter fnr(repressor) plus RBS_AmilCP+Term_PSB1C3
| |
| - | *Well 4: clone4 of ligation promoter fnr(repressor) plus RBS_AmilCP+Term_PSB1C3
| |
| - |
| |
| - | *Gel : 1.0%
| |
| - | |}
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - |
| |
| - | :<u>Ligation</u>
| |
| - |
| |
| - |
| |
| - |
| |
| - | :For promoter fnr(repressor)+ RBS_LacZ+Term_PSB1C3 :
| |
| - |
| |
| - | {| border="1" align="center"
| |
| - | |-
| |
| - | |promoter fnr(repressor)
| |
| - | |8µl
| |
| - | |-
| |
| - | |RBS_LacZ+Term
| |
| - | |3µl
| |
| - | |-
| |
| - | |Ligation buffer
| |
| - | |2µl
| |
| - | |-
| |
| - | |Ligation T4 enzyme
| |
| - | |2µl
| |
| - | |-
| |
| - | |H2O
| |
| - | |6µl
| |
| - | |}
| |
| - |
| |
| - | Transformation theligation and spread in LB plates with Chlorenphenicol and Xgal.
| |
| - | incubated at 37°C in aerobic condition over night.
| |
| - |
| |
| - | :For promoter fnr(activator)nirK + RBS_LacZ+Term_PSB3K3 :
| |
| - |
| |
| - |
| |
| - | {| border="1" align="center"
| |
| - | |-
| |
| - | |promoter fnr(activator)nirK
| |
| - | |3µl
| |
| - | |-
| |
| - | |RBS_LacZ+Term
| |
| - | |3µl
| |
| - | |-
| |
| - | |Plasmid PSB3K3
| |
| - | |5µl
| |
| - | |-
| |
| - | |Ligation buffer
| |
| - | |2µl
| |
| - | |-
| |
| - | |Ligation T4 enzyme
| |
| - | |1µl
| |
| - | |-
| |
| - | |H2O
| |
| - | |6µl
| |
| - | |}
| |
| - |
| |
| - | Transformation theligation and spread in LB plates with k and Xgal.
| |
| - | incubated at 37°C in anaerobic condition over night.
| |
| - |
| |
| - |
| |
| - | :For promoter fnr(repressor) + RBS_LacZ+Term_PSB3K3 :
| |
| - |
| |
| - |
| |
| - | {| border="1" align="center"
| |
| - | |-
| |
| - | |promoter fnr(repressor)
| |
| - | |3µl
| |
| - | |-
| |
| - | |RBS_LacZ+Term
| |
| - | |3µl
| |
| - | |-
| |
| - | |Plasmid PSB3K3
| |
| - | |5µl
| |
| - | |-
| |
| - | |Ligation buffer
| |
| - | |2µl
| |
| - | |-
| |
| - | |Ligation T4 enzyme
| |
| - | |1µl
| |
| - | |-
| |
| - | |H2O
| |
| - | |6µl
| |
| - | |}
| |
| - |
| |
| - | Transformation theligation and spread in LB plates with k and Xgal.
| |
| - | incubated at 37°C in aerobic condition over night.
| |
| - |
| |
| - |
| |
| - | {| border="1" align="center"
| |
| - | ||<big>Previous week</big>
| |
| - |
| |
| - | |[[Team:Paris_Saclay/Notebook|<big>Back to calendar</big>]]
| |
| - |
| |
| - | |[[Team:Paris Saclay/Notebook/August/30|<big>Next day</big>]]
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | {{Team:Paris_Saclay/incl_fin}}
| |
| - |
| |
| | {{Team:Paris_Saclay/incl_debut_generique}} | | {{Team:Paris_Saclay/incl_debut_generique}} |
| | | | |
| - | ='''Notebook : August 23'''= | + | ='''Notebook : August 30'''= |
| | | | |
| | =='''Lab work'''== | | =='''Lab work'''== |
Notebook : August 30
Lab work
A - Aerobic/Anaerobic regulation system
Objective : characterize Bba_K1155000, Bba_K1155004, Bba_K1155005, Bba_K1155006
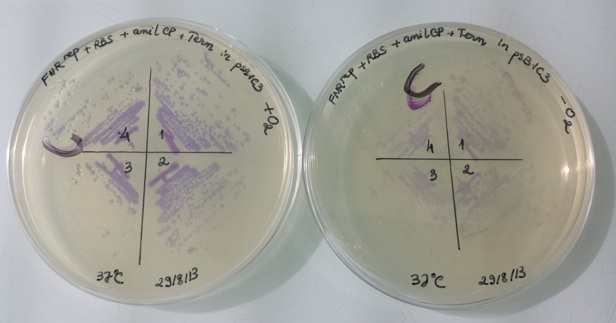
1 - Results of liquid culture of Pfnr-RBS-Amil CP-Term in PSB1C3 in aerobic and anaerobic conditions
XiaoJing

In anaerobic conditions, thank to promotor RBS-Amil CP-Term, the dectetion gene is activated and we can see a violet coloration of bacterias.
In aerobic conditions, promotor RBS-Amil CP-Term is inactive, the dectetion gene isn't activated and we can't see any coloration of bacterias.
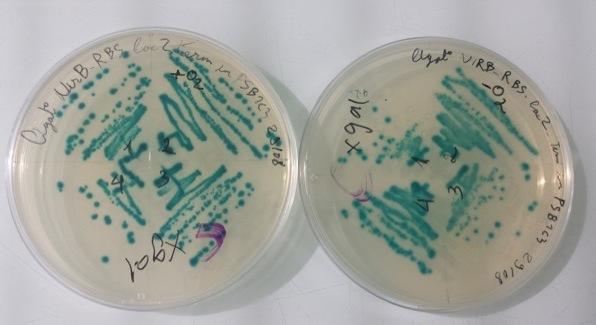
2 - Results of culture of Pfnr-RBS-Amil CP-Term in PSB1C3, NirB with RBS-LacZ-Term in PSB1C3 in aerobic and anaerobic conditions
XiaoJing
|
Purification of 08/22/13 didn't work. We have blue colonies for Pfnr with RBS-Amil CP-Term in PSB1C3 in aerobic and anaerobic conditions. We also have blue colonies for NirB with RBS-LacZ-Term in PSB1C3 in anaerobic conditions.
|
3 - Ligation of Pfnr with RBS-LacZ-Term in PSB1C3
XiaoJing
Used quantities :
- Pfnr : 8µL
- RBS-LacZ-Term in PSB1C3 : 3µL
- Buffer ligation : 2µL
- Ligase : 1µL
- H2O : 6µL
4 - Transformation of ligation of Pfnr with RBS-LacZ-Term in PSB1C3 in DH5α
XiaoJing
Protocol : Bacterial transformation
We incubate it at 37°C with Xgal in aerobic conditions.
((((((((((((((====Objective : obtaining Pfnr, NarK, NarG or NirB and RBS-LacZ-Term or RBS-AmilCP-Term in PSB3K3====
1 - Ligation of Pfnr with RBS-LacZ-Term in PSB3K3 and NarK with RBS-LacZ-Term in PSB3K3
XiaoJing
Used quantities :
- Pfnr, NarK : 3µL
- RBS-LacZ-Term : 3µL
- PSB3K3 : 5µL
- Buffer ligase : 2µL
- Ligase : 1µL
- H2O : 6µL
2 - Transformation of ligation of Pfnr with RBS-LacZ-Term in PSB3K3 and NarK with RBS-LacZ-Term in PSB3K3 in DH5α
XiaoJing
Protocol : Bacterial transformation
We incubate it at 37°C with Xgal in anaerobic conditions for NarK with RBS-LacZ-Term in PSB3K3. We incubate it at 37°C with Xgal in aerobic conditions for NarK with Pfnr-LacZ-Term in PSB3K3.
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining FNR and BphR2 proteins
1 - Electrophoresis of Colony PCR of FNR, RBS-FNR and RBS-BphR2
| ]]
|
- Well 1 : 6µL DNA Ladder
- Well 2 to 17 : 5µL of FNR+1µl of 6X loading dye
- Well 18 to 26 : 5µL of RBS-BphR2+1µl of 6X loading dye
- Well 27 : 6µL DNA Ladder
- Well 28 to 41 : 5µL of RBS-FNR+1µl of 6X loading dye
- Well 42 to 49 : 5µL of RBS-BphR2+1µl of 6X loading dye
- WEll 50 : 6µL DNA Ladder
- Gel : 1%
|
Expect sizes :
- FNR : 1096 bp
- RBS-FNR : 1014 bp
- RBS-BphR2 : 1469 bp
|
We obtain fragment at right size for FNR and RBS-FNR. The Gisbon assembly of 08/26/13 was good.
|
 "
"


