|
Our Project
What is MicroBeagle I hear you cry?
Well, MicroBeagle is a E.coli cell that has been modified so that it can “hunt down” pathogens. Just like a Beagle, the hunting dog, searches and finds rabbits and hares, our MircoBeagle finds pathogens and detects them through physical binding! By utilising the Cpx pathway and GFP we hope to create a new biosensor that gives results that can be seen with the naked eye.
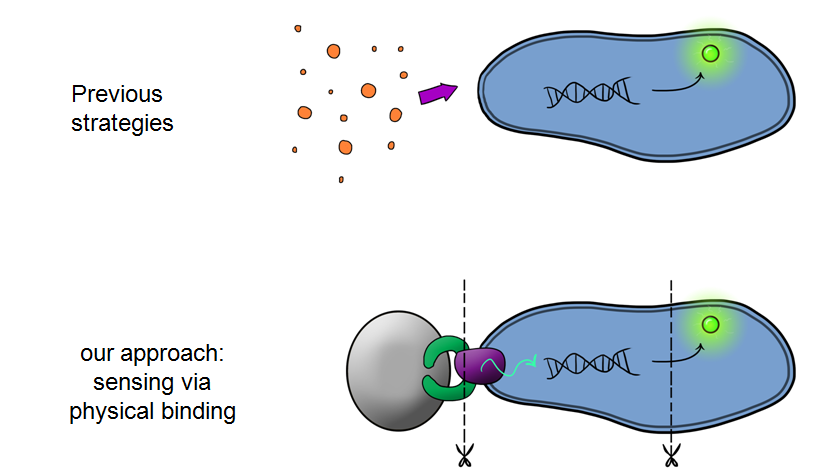
MicroBeagle is different to anything iGEM has ever seen! Our goal was to develop a modular, general platform for pathogen detection. What makes Leeds iGEM's MicroBeagle distinctive to previous strategies used for particle detection within iGEM is that we used physical binding in order to detect our "pathogen", whereas the previous iGEM teams used a signal in solution to detect their particles.
Why is the MircoBeagle a useful device?
There could be many different possible uses for our device as the pathogen detection mechanism we are using is modular and can easily be tailored to individual needs. This is achieved by swapping out the gene coding the binding domain to suit your need. We see the MircoBeagle being used for testing blood samples, detection of heavy metals and, our main focus, detection of pathogens in water samples.
Why Water Testing is so important?
Detecting pathogens in water is a really important application as 3.4 million people a year die from water related diseases.
768 million people in the world do not have access to safe water. This is roughly one in ten of the world's population, the majority of which is caused by faecal contamination: poor sewage systems that flow back into the water supply
The issue is actually more complex and subtle than just direct health problems, the knock on economic problems that result from poor sanitation are phenomenal
Lack of water, sanitation and hygiene costs Sub-Saharan African countries more in lost GDP than the entire continent gets in development aid
The figures are shocking and we as a team want to create something that may, in the future, help to reduce those numbers.
So our challenge was to address this issue using a novel synthetic biological approach, hence the MircoBeagle was born!
Below is a summary of our Biosystem development strategy...
Phase I
We split our project into self-contained phases, to help better organise ourselves, but also lay out a road map for our own future work within and without iGEM. The first of these looks at developing, characterising and producing basic Biosystems.
Biosystem 1
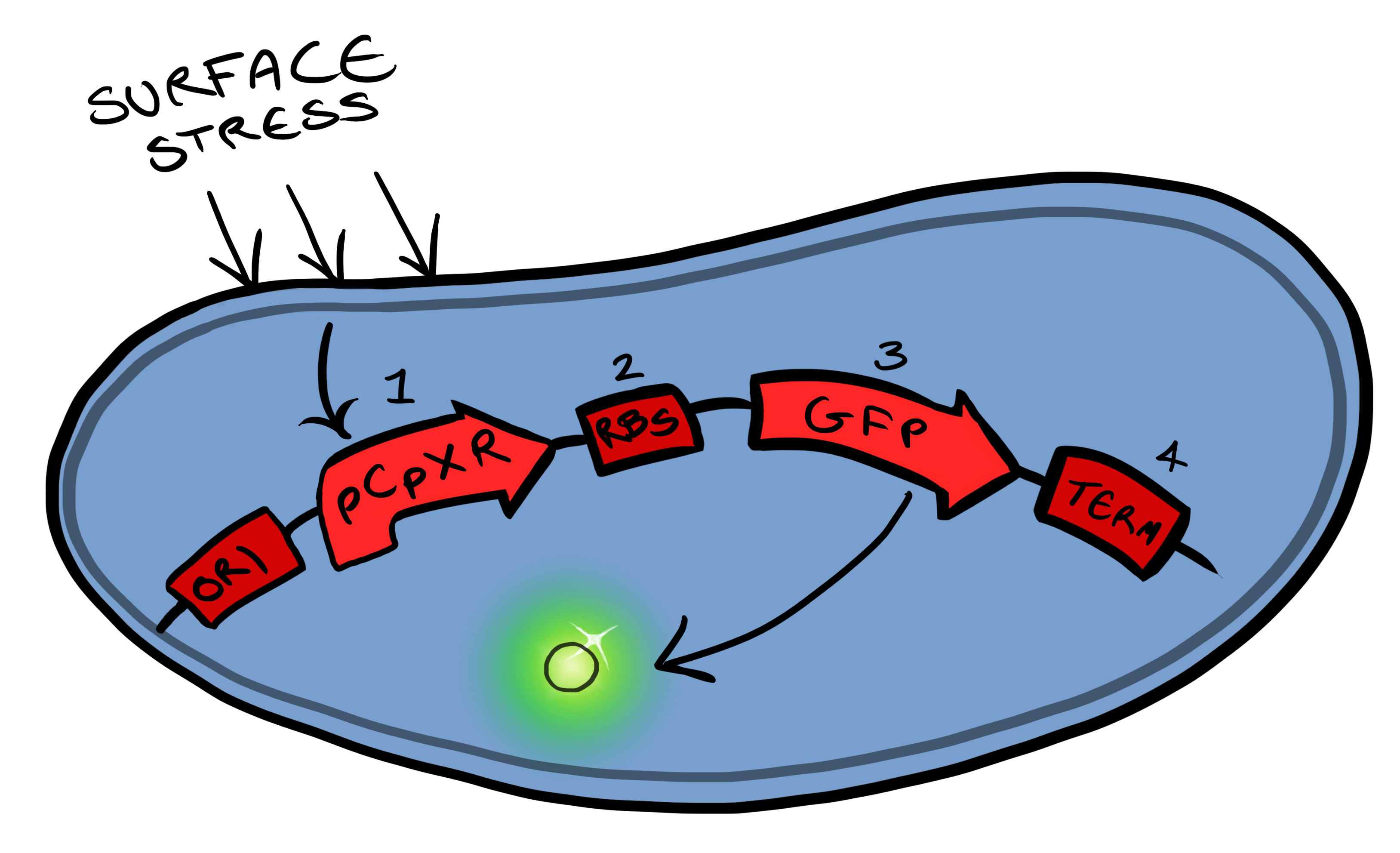
Biosystem 1 is a very simple modification of the Cpx promoter in E. coli to produce a fluorescent reporter when the cell is under membrane stress. We will then characterise and test the insertion following the work by [http://www.ncbi.nlm.nih.gov/pubmed/11830644 Otto & Silhaevy 2001] in which glass beads were used to induce hydrophobic membrane stress. What we would hope is that when we add our cells to a vial containing similar hydrophobic beads we can detect fluorescence emissions confirming that our biobrick is working as we had hoped. We know that membrane stress can be caused by many stimuli, so we have also looked at the effect different membrane stresses cause on the florescence achieved; these membrane stresses include, pH, temperture and detergent concentration.
Bio bricks we used to assemble Biosystem 1 are:
BBa_K135000 pCpxR promoter responds to membrane stress
BBa_K081012 Green fluorescent protein generator. Contain ribosome binding site and terminator
Biosystem 2
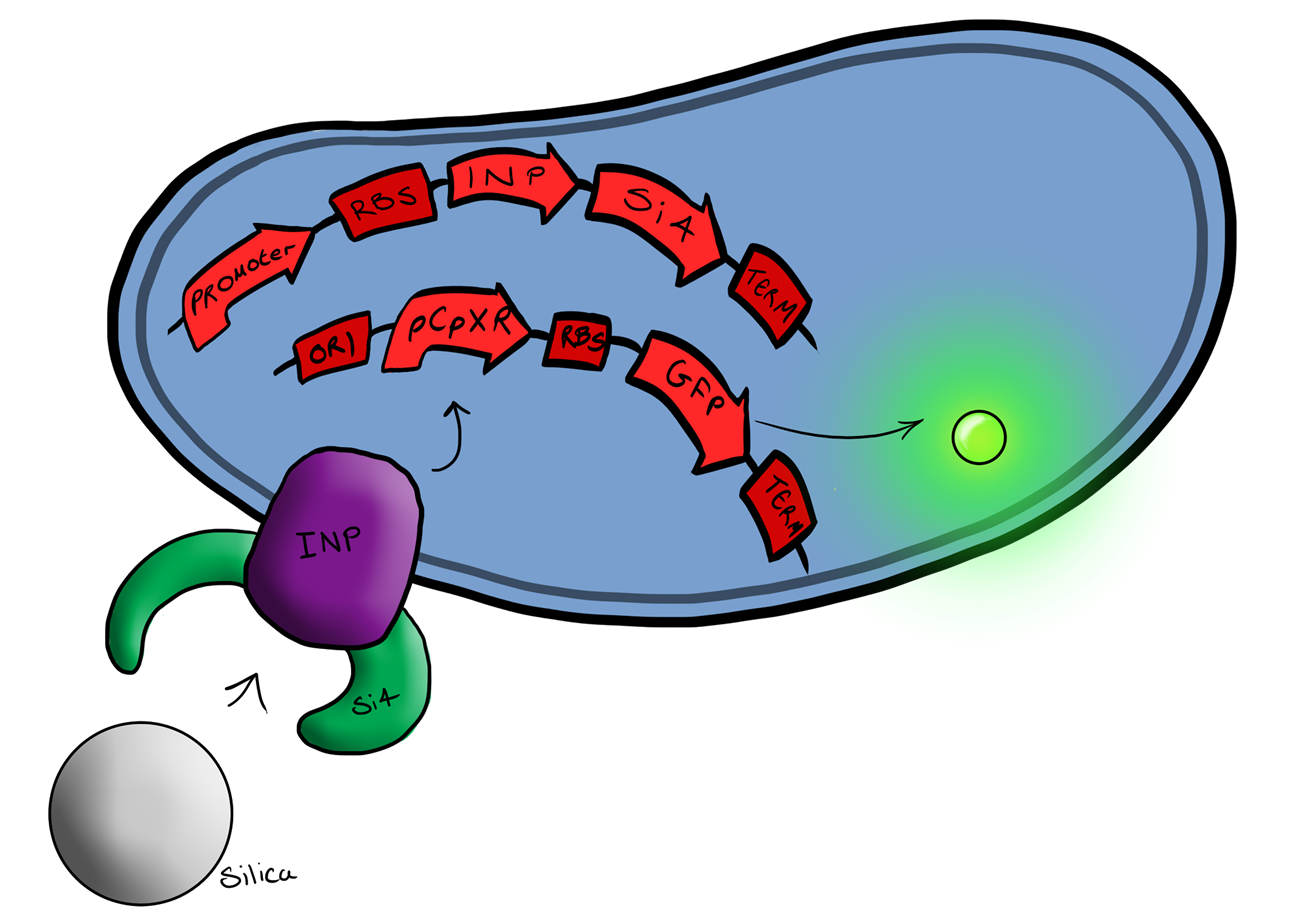
 Simultaneously, we will be developing a Biosystem that is suited for physical attachment and detection of particles. This will work using Ice Nucleation Protein (INP) to display a oligo-peptide of our choice on the outer-membrane of our E. coli, initially this will be a peptide capable of binding silica beads allowing us to create a model system of pathogen detection. INP is a transmembrane protein that expresses any sequence that is placed on the C-terminus of its gene on the outer surface of the cell.
The cells producing this receptor complex will also be constitutively producing a fluorescent reporter, GFP, to allow for its detection during the characterisation experiments but this time the focus of Biosystem 2 is on the adhesion proteins being used.
The Biobricks we used for this Biosystem are:
BBa_K081012 for the Green fluorescent protein generator which contain a ribosome binding site and terminator.
BBa_K523008 is the Ice nucleation protein any sequence added to the C-terminus of the INP will be transported to cell membrane and be displayed on the surface of the cell.
BBaa_B0015, which is a double terminator,
BBa_B0034 a strong ribosome binding site and finally
BBa_J23119 a Constitutive promoter
Phase II
Phase II takes the products of Phase I to the next level, and begins to look at integration of the two BioBricks.
Biosystem 3
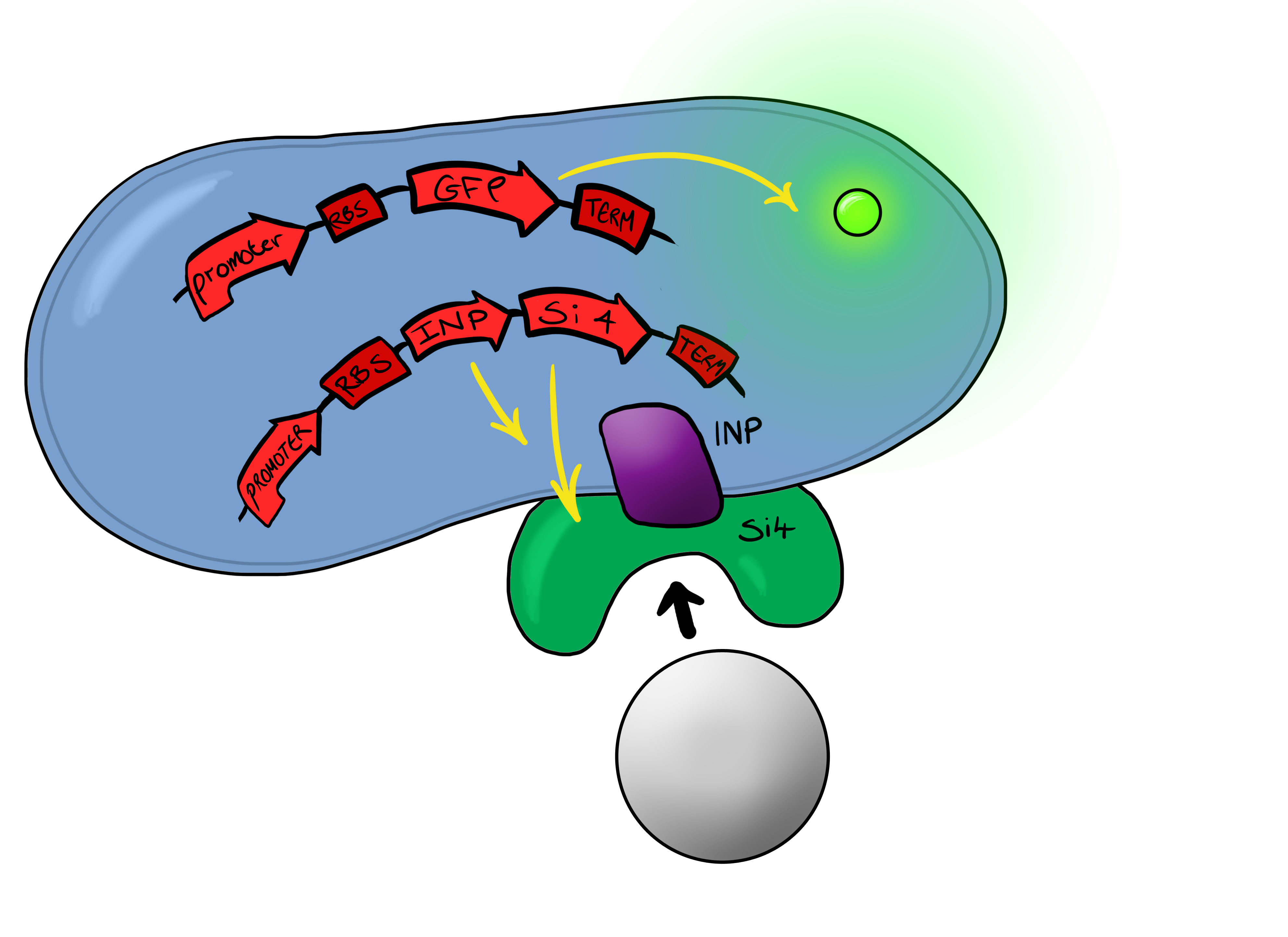
Biosystem 3 brings together BS1 and BS2 in one plasmid; the genes that code for the INP and the Si4 binding domain are now located on the same plasmid as the CpxP promoter and the GFP generator. The effect of this is that when the silca bead binds to the Si4 binding domain connected to the INP protein and causes surface stress the CpxP promoter will be induced. This will cause GFP to be produced and this will act as a visible signal that the 'pathogen' has been detected.
This is the final MircoBeagle device and has the potential to be made into many different pathogen detectors. This can easily be done by swapping out the gene coding for the binding moiety next to the INP gene. Once Biosystem 3 has been suitably characterised and all bugs fixed, the next step will be to swap the silca binding domain for a binding domain that binds to a particular pathogen.
The Cpx Pathway
This project is highly dependent upon a naturally occuring pathway in E.Coli called the Cpx pathway. It is associated with the regulation of periplasmic membrane stress and the misfolding of surface proteins. We may need to fine tune MicroBeagle for different applications, so by understanding the way the pathway is regulated, we stand a better chance of controlling the exact response we want. This may be done in a number of ways, from utilisation of an off-switch regulator, CpxP, to additonal pathways used to create a bio-logic gate or even by optimisation of buffer solution.
A brief explanation of the Cpx Pathway
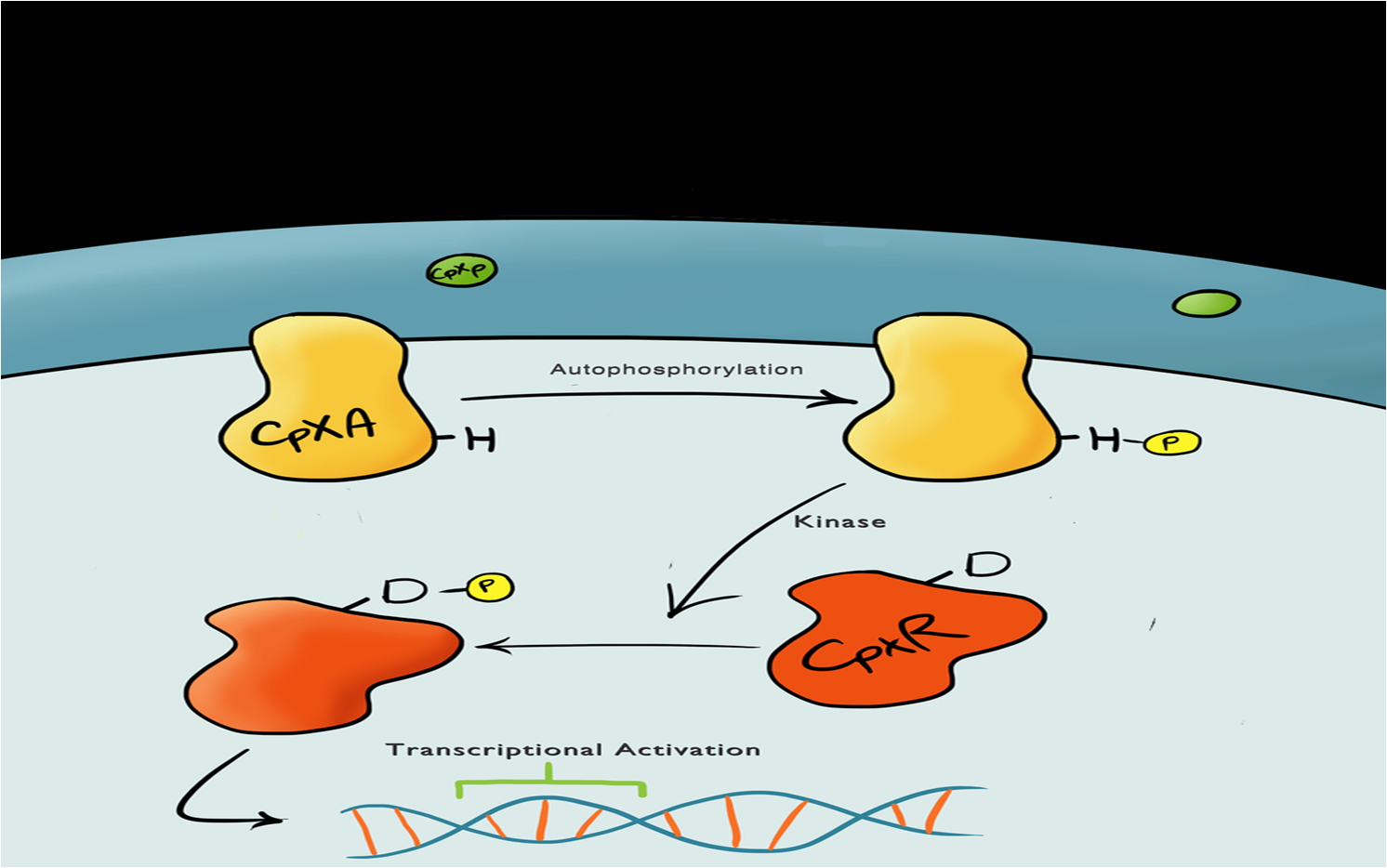
The Cpx pathway responds to various membrane stress; it is a two component signal transduction pathway. The first component is CpxA, an inner membrane protein which, when bound to CpxP (an inhibitor molecule), in inactive. When CpxP is unbound from CpxA, CpxA autophosphorylates itself. A kinase enzyme then phosphorylates the CpxR, the second component in the pathway, which then binds to the Cpx promoter and activates transcription.
There is still a lot of debate regarding this pathway and many different theories but it is thought that membrane stress causes the misfolding of proteins (pili being the main contributor) causes misfolded subunits to accumulate in the periplasm and titrate the CpxP inhibitor molecule off the CpxA and activate the pathway.
|  "
"