Team:TU-Eindhoven/KillingMechanism
From 2013.igem.org
(→The Kill Switch in Detail: The Ganciclovir /Thymidine Kinase System) |
(→The Alternative System: The 5-Fluorocytosine/ Cytosine Deaminase System) |
||
| Line 31: | Line 31: | ||
==The Alternative System: The 5-Fluorocytosine/ Cytosine Deaminase System== | ==The Alternative System: The 5-Fluorocytosine/ Cytosine Deaminase System== | ||
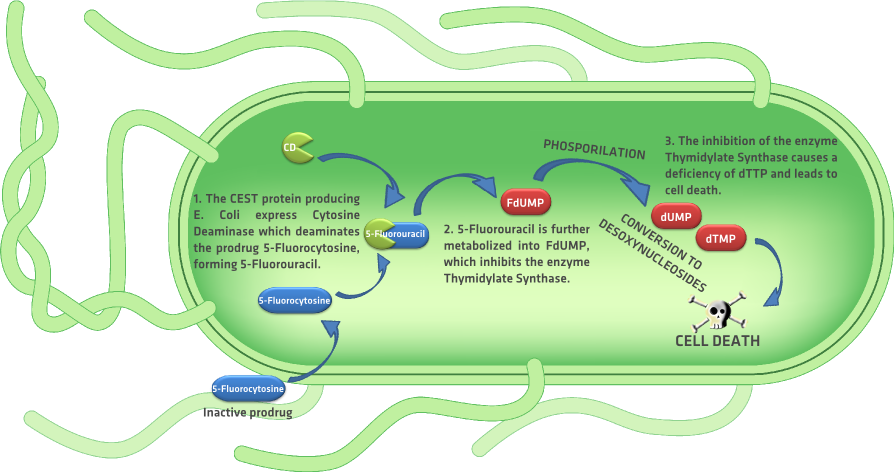
| - | {{:Team:TU-Eindhoven/Template:Float | position=right | size= | + | Similar to the GCV/HSV-TK GDEPT system, is the Escherichia coli cytosine deaminase (CD) enzyme with prodrug 5-fluorocytosine. Like the prodrug ganciclovir, systemic administration of the prodrug 5-fluorocytosine induces apoptosis only in the cells that express the cytosine deaminase enzyme. Cell death is induced when the CD deaminates the prodrug 5-fluorocytosine, forming 5-fluorouracil {{:Team:TU-Eindhoven/Template:Figure | id=kmIIFigure }}. 5-fluorouracil is then metabolized into 5-fluoro-28-deoxyuridine-58-monophosphate (FdUMP), 5- fluoro-28-deoxyuridine-58-triphosphate (FdUTP), and 5- fluorouridine-58-triphosphate (FUTP). FdUMP inhibits the enzyme thymidylate synthetase which converts dUMP into thymidylate (dTMP). Because thymidylate can only be produced de novo by the enzyme thymidylate synthetase, the inhibition of the enzyme eventually generates a deficiency of dTTP, causing the incorporation of FdUTP into the DNA and with it cell death. {{:Team:TU-Eindhoven/Template:RefAgain | id=aghiEtAlGCVFlu }} Given that the chasis of our device is Escherichia coli, the implementation of the CD/5’fluorocytosine system on our device could be an alternative to the GCV/HSV-TK system. We didn’t explore this alternative further but we will consider it when testing the kill switch in vitro in case the GCV/HSV-TK is not successful in inducing apoptosis in our CEST protein producing bacteria. |
| + | |||
| + | {{:Team:TU-Eindhoven/Template:Float | position=right | size=12 }} | ||
{{:Team:TU-Eindhoven/Template:Image | filename=killMechanismII.png}} | {{:Team:TU-Eindhoven/Template:Image | filename=killMechanismII.png}} | ||
{{:Team:TU-Eindhoven/Template:FloatEnd | caption=Apoptosis induced by the 5-Fluorocytosine/ Cytosine Deaminase system in the CEST protein producing bacteria. | id=kmIFigure }} | {{:Team:TU-Eindhoven/Template:FloatEnd | caption=Apoptosis induced by the 5-Fluorocytosine/ Cytosine Deaminase system in the CEST protein producing bacteria. | id=kmIFigure }} | ||
| - | |||
| - | |||
==References== | ==References== | ||
Revision as of 18:09, 22 September 2013



Contents |
The Kill Switch as a Safety Lock

This means that by implementing this controllable safety lock we can inactivate our bacterial device at any point of the MR imaging process by administering Ganciclovir to the patient.
The Kill Switch in Detail: The Ganciclovir /Thymidine Kinase System

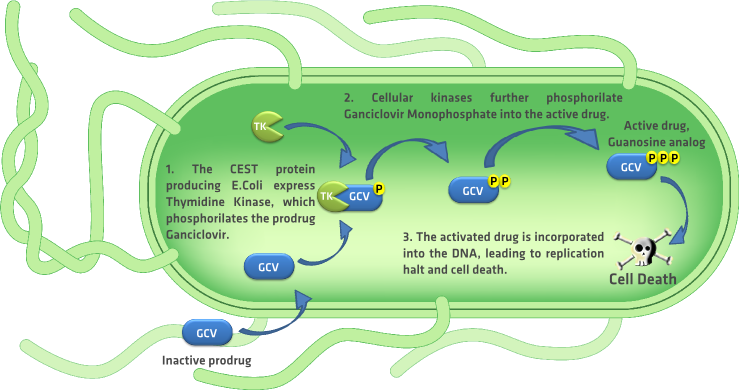
A rapidly evolving research area in the field of oncology is the one focused on bacterial based cancer therapies. In this type of therapies, bacteria like Escherichia Coli and Salmonella typhimurium are used as tumor targeting systems, able to invade tumors and induce cytotoxicity in the tumor. These bacteria are used to deliver cytotoxic agents, immunostimulatory cytokine, and genetic material to enable host-cell production of these molecules. forbesBactN.S. Forbes, Engineering the perfect(bacterial) cancer therapy. Nature , 785-794 (2010) Using the bacteria for the bringing genetic material to the tumor cells is similar to the suicide gene therapy, also known as gene-directed enzyme prodrug therapy (GDEPT), which is widely used in cancer treatment. BugajNextGenTherL.J. Bugaj, D.V. Schaffer, Bringing next-generation therapeutics to the clinic through synthetic biology. Current Opinion in Chemical Biology 16, 355-361 (2012) One of the best characterized and used GDEPT systems is the herpes simplex virus thymidine kinase (HSV-TK) enzyme with purine nucleoside analog ganciclovir (GCV) as a prodrug. Systemic administration of the prodrug ganciclovir induces apoptosis only in the cells that express the HSV-thymidine kinase enzyme, sparing the life of the cells that don’t express this enzyme. To induce apoptosis, the HSV-TK phosphorylates ganciclovir to form ganciclovir-monophosphate, which is further phosphorylated by cellular kinases, forming ganciclovir triphosphate.aghiEtAlGCVFluM. Aghi, C.M. Kramm, T.C. Chou, X.O. Breakefield, E.A. Chiocca, Synergistic Anticancer Effects of Ganciclovir/Thymidine Kinase and 5-Fluorocytosine/Cytosine Deaminase Gene Therapies. Journal of the National Cancer Institute 90, 370-380 (1998) Ganciclovir triphosphate inhibits cellular DNA polymerase and is incorporated into the DNA, inhibiting the DNA synthesis and consequently leading to apoptosis. depicts the complete process of cell death that was previously described.
We propose to use this system as our killing mechanism, considering that it is well characterized and used in applications related to the principle of our device. However at this point of our project the in vitro implementation of this system is not possible and thus we focused on a pharmacokinetics model of the prodrug distribution in the body and the elimination of the CEST protein producing bacteria from the system. The following section describes the the main results from the pharmacokinetics model.
The TK/GCV System: Implementation
We developed a pharmacokinetics model of the prodrug Ganciclovir distribution and the systemic elimination of the CEST protein production device. The complete results can be found in the Drylab section of our wiki, but here we present a summary of the most important conclusions.
- Elimination rate
- Ganciclovir distribution
The Alternative System: The 5-Fluorocytosine/ Cytosine Deaminase System
Similar to the GCV/HSV-TK GDEPT system, is the Escherichia coli cytosine deaminase (CD) enzyme with prodrug 5-fluorocytosine. Like the prodrug ganciclovir, systemic administration of the prodrug 5-fluorocytosine induces apoptosis only in the cells that express the cytosine deaminase enzyme. Cell death is induced when the CD deaminates the prodrug 5-fluorocytosine, forming 5-fluorouracil . 5-fluorouracil is then metabolized into 5-fluoro-28-deoxyuridine-58-monophosphate (FdUMP), 5- fluoro-28-deoxyuridine-58-triphosphate (FdUTP), and 5- fluorouridine-58-triphosphate (FUTP). FdUMP inhibits the enzyme thymidylate synthetase which converts dUMP into thymidylate (dTMP). Because thymidylate can only be produced de novo by the enzyme thymidylate synthetase, the inhibition of the enzyme eventually generates a deficiency of dTTP, causing the incorporation of FdUTP into the DNA and with it cell death. aghiEtAlGCVFlu Given that the chasis of our device is Escherichia coli, the implementation of the CD/5’fluorocytosine system on our device could be an alternative to the GCV/HSV-TK system. We didn’t explore this alternative further but we will consider it when testing the kill switch in vitro in case the GCV/HSV-TK is not successful in inducing apoptosis in our CEST protein producing bacteria.
References
 "
"



