Team:Duke/Project/Design
From 2013.igem.org
Hyunsoo kim (Talk | contribs) (→Experimental Design) |
Hyunsoo kim (Talk | contribs) (→Experimental Design) |
||
| Line 21: | Line 21: | ||
<div align="center"> Figure 1.3.1. Experimental Design: Cooperative repression using TALEs</div><br><br> | <div align="center"> Figure 1.3.1. Experimental Design: Cooperative repression using TALEs</div><br><br> | ||
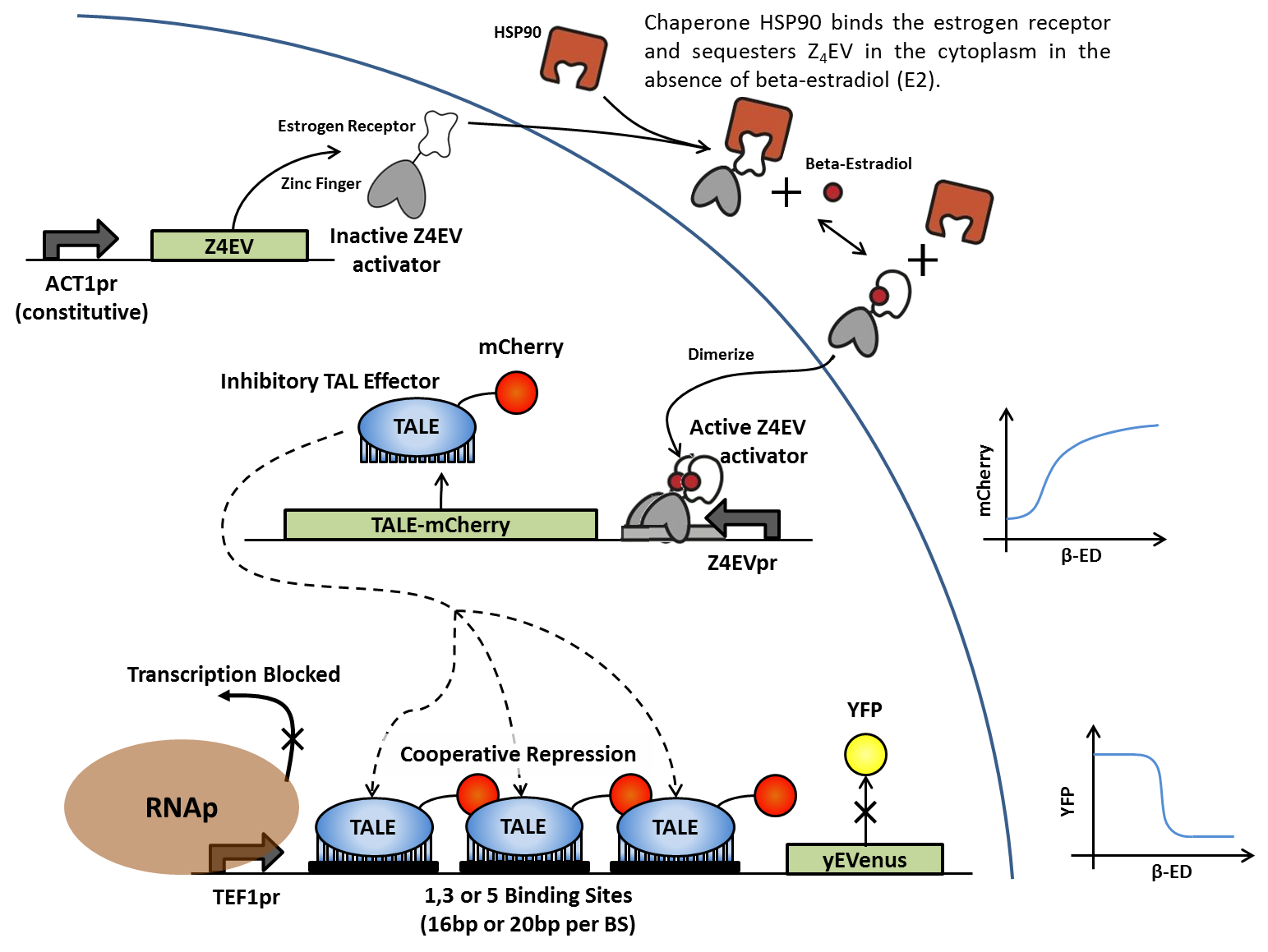
| - | + | In the absence of β-estradiol, the Z4EV zinc finger protein is sequestered in the cytoplasm by HSP90. Once B-estradiol is added, the Z4EV protein dissociates from the HSP90, revealing a nuclear localization signal (NLS) that translocates the protein to the nucleus and activating the Z4EV promoter. Activation of the Z4EV promoter activates transcription of the iTAL-mcherry fusion protein, which in turn binds to artificial DNA sequences in front of the yEVenus reporter gene. Binding of the iTAL to multiple sites between the constitutive promoter and the reporter gene blocks transcription by RNA polymerase and suppresses yEVenus levels. Multiple variations of this construct differ in binding sequence length and content, as well as number of tandem binding sites in order to generate cooperativity. iTALs are designed to match specific binding sites. | |
| - | + | ||
<br><br><br> | <br><br><br> | ||
| Line 28: | Line 27: | ||
<div align="center"> Figure 1.3.2. Experimental Design: Cooperative repression using CRISPRs</div> <br><br> | <div align="center"> Figure 1.3.2. Experimental Design: Cooperative repression using CRISPRs</div> <br><br> | ||
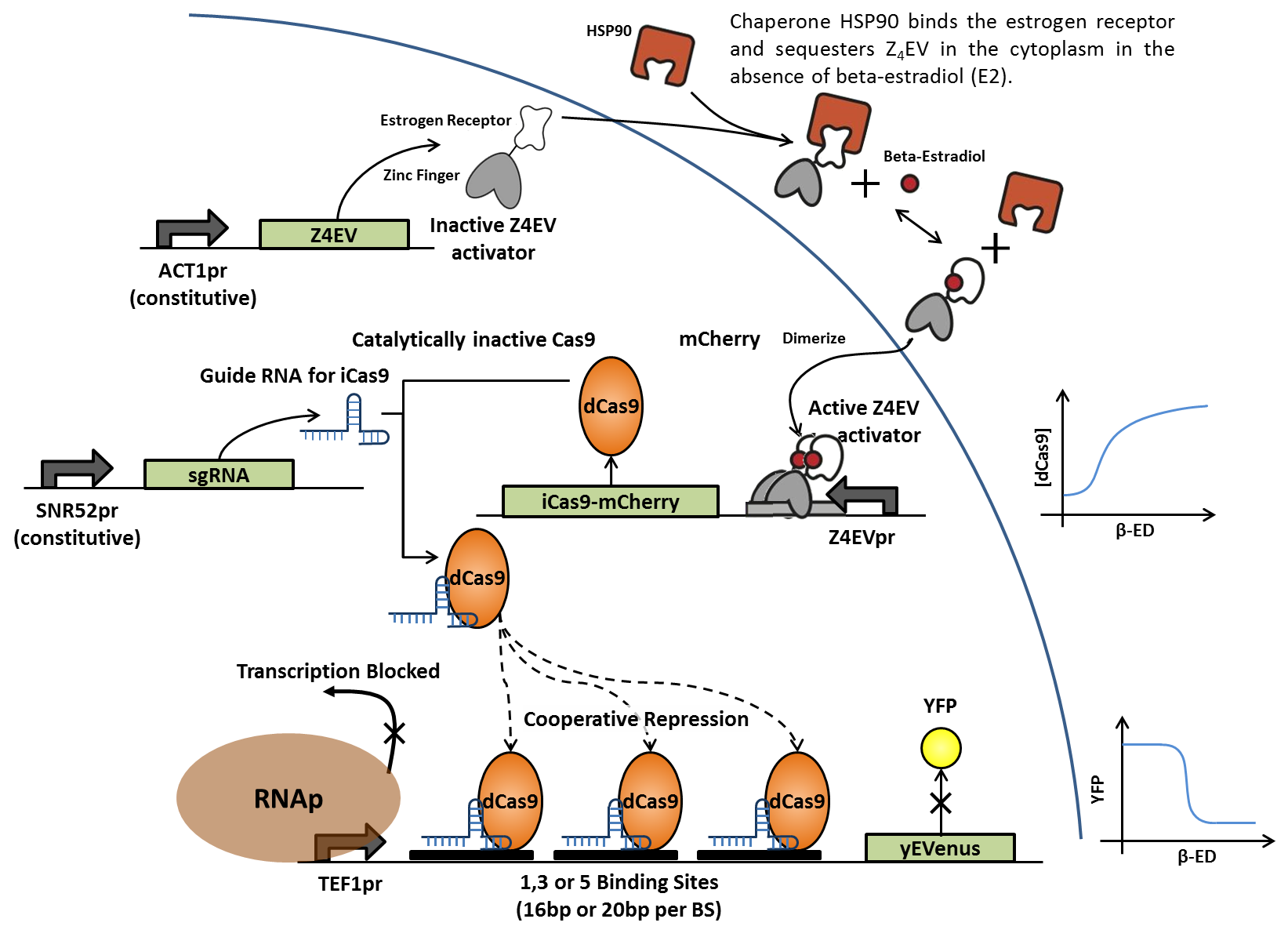
| - | + | In the same fashion as above, addition of β-estradiol activates transcription of dCas9-mCherry fusion protein, which associates with constitutively expressed, sequence-specific small guide RNAs (sgRNAs). The RNA-protein complex then binds to artificial DNA sequences in front of the yEVenus reporter gene. Binding of the complex to multiple sites between the constitutive promoter and the reporter gene blocks transcription by RNA polymerase and suppresses yEVenus levels. Multiple variations of this construct differ in binding sequence length and content, as well as number of tandem binding sites in order to generate cooperativity. Each sgRNAs is designed to match a specific enhancer sequence. | |
| - | + | <br><br> | |
<div align="center"> | <div align="center"> | ||
[[File:MutualTale.png|350px]][[File:MutualCas9.png|350px]] </div> | [[File:MutualTale.png|350px]][[File:MutualCas9.png|350px]] </div> | ||
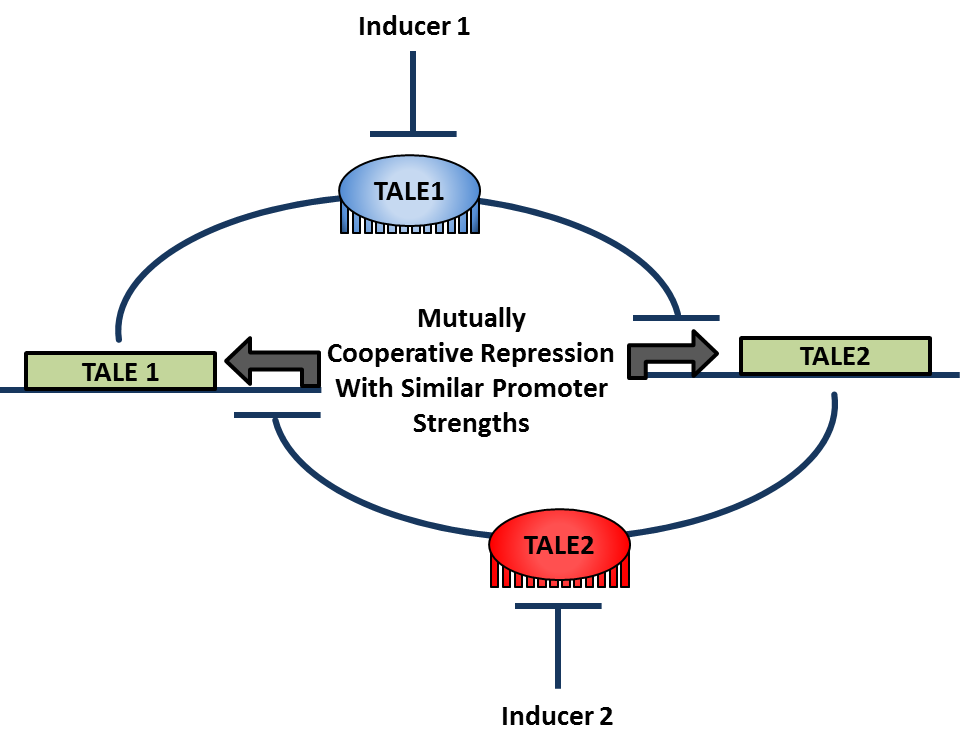
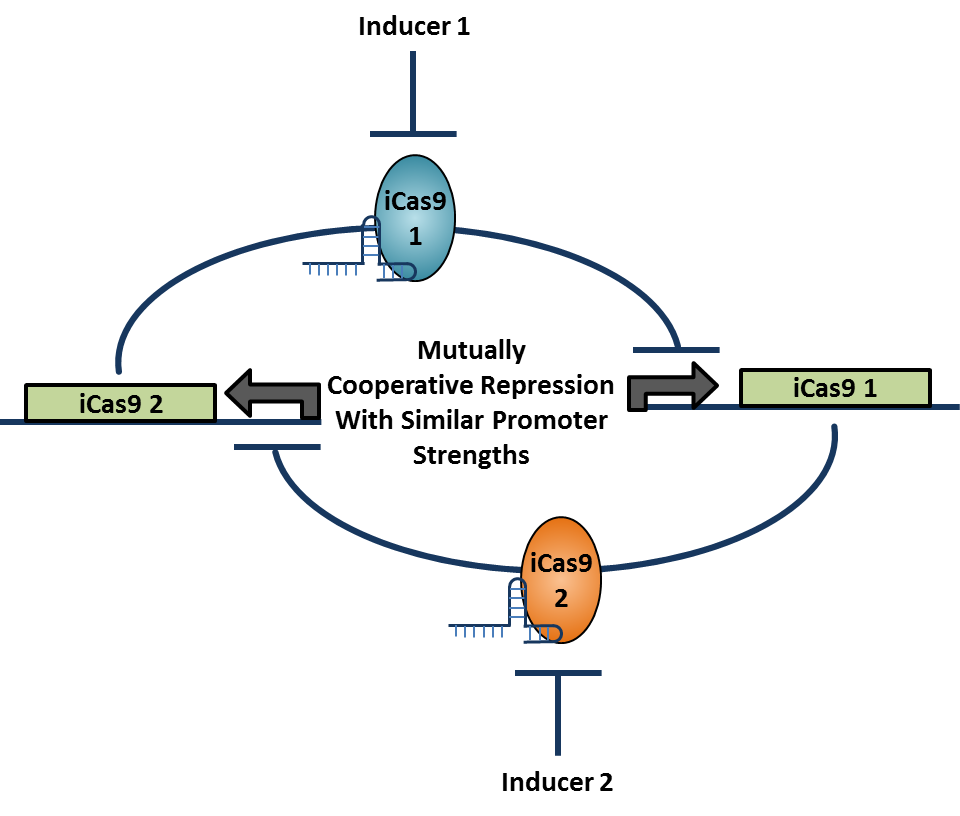
<div align="center"> Figure 1.3.3. Toggle Switch Design with Two Mutually Repressive Genes</div> <br><br> | <div align="center"> Figure 1.3.3. Toggle Switch Design with Two Mutually Repressive Genes</div> <br><br> | ||
| + | Write stuff here | ||
| + | |||
| + | |||
| + | <br><br> | ||
===References=== | ===References=== | ||
Revision as of 18:57, 27 September 2013
Experimental Design
- Design enhancer sequences and corresponding artificial transcription factors (ATFs). Check constructs against the yeast genome and transcription factor sequences to ensure independence of the system and limit off-target effects.
- Construct two plasmid libraries: one containing fluorescent reporter genes driven by constitutive promoters, and one containing artificial transcription factor genes (TALEs, dCas9, and sgRNAs) driven by inducible promoters. Enhancer sequences to match each ATF were inserted between the promoter and the gene in the reporter constructs.
- Integrate plasmids into the yeast genome, in pairs of corresponding reporters and ATFs. Use flow cytometry along with control strains to quantifiably test for repressive activity and cooperativity in ATF-enhancer interaction.
- Select compatible sets of ATF-enhancer pairs, using mathematical modeling results to determine the most robust circuit designs. Design, integrate, and test these circuits for balanced cooperative repression and bistability.
In the absence of β-estradiol, the Z4EV zinc finger protein is sequestered in the cytoplasm by HSP90. Once B-estradiol is added, the Z4EV protein dissociates from the HSP90, revealing a nuclear localization signal (NLS) that translocates the protein to the nucleus and activating the Z4EV promoter. Activation of the Z4EV promoter activates transcription of the iTAL-mcherry fusion protein, which in turn binds to artificial DNA sequences in front of the yEVenus reporter gene. Binding of the iTAL to multiple sites between the constitutive promoter and the reporter gene blocks transcription by RNA polymerase and suppresses yEVenus levels. Multiple variations of this construct differ in binding sequence length and content, as well as number of tandem binding sites in order to generate cooperativity. iTALs are designed to match specific binding sites.
In the same fashion as above, addition of β-estradiol activates transcription of dCas9-mCherry fusion protein, which associates with constitutively expressed, sequence-specific small guide RNAs (sgRNAs). The RNA-protein complex then binds to artificial DNA sequences in front of the yEVenus reporter gene. Binding of the complex to multiple sites between the constitutive promoter and the reporter gene blocks transcription by RNA polymerase and suppresses yEVenus levels. Multiple variations of this construct differ in binding sequence length and content, as well as number of tandem binding sites in order to generate cooperativity. Each sgRNAs is designed to match a specific enhancer sequence.
Write stuff here
References
- McIsaac R.S, Oakes B.L, Wang X, Dummit K.A, Botstein D, Noyes M.B: Synthetic gene expression perturbation systems with rapid, tunable, single-gene specificity in yeast. Nuc Ac Res 2012, 1-10.
- Murphy K.F, Balazsi G, Collins J.J: Combinatorial promoter design for engineering noisy gene expression. Proc. Nat. Acad. Sci. USA 2007, 104(31):12726–12731.
- Qi L.S, Larson M.H, Gilbert L.A, Doudna J.A, Weissman J.S, Arkin A.P, Lim W.A: Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013, 152:1173-1183.
 "
"