Team:Paris Saclay/Modeling/FNR
From 2013.igem.org
(→Conclusion) |
(→Conclusion) |
||
| Line 145: | Line 145: | ||
=='''Conclusion'''== | =='''Conclusion'''== | ||
<p>Our model reveal successfully the different states of FNR regulation. As you can see, in a anaerobic condition, the number of FNR active is significantly raised, so do the number of green reporter protein. In aerobic condition, because of the presence of oxygen, FNR inactive dominates in the model, the production of green ceases so the number of green protein drops, at the same time the repression for the red reporter disappear so production of red protein restart.</p> | <p>Our model reveal successfully the different states of FNR regulation. As you can see, in a anaerobic condition, the number of FNR active is significantly raised, so do the number of green reporter protein. In aerobic condition, because of the presence of oxygen, FNR inactive dominates in the model, the production of green ceases so the number of green protein drops, at the same time the repression for the red reporter disappear so production of red protein restart.</p> | ||
| - | <p>Back to our experiments, we used LacZ (blue color BBa_K11550007) and AmilCP (pink color BBa_K11550003) as expression | + | <p>Back to our wet experiments, we used LacZ (blue color BBa_K11550007) and AmilCP (pink color BBa_K11550003) as 2 independent expression reporters which correspond to green reporter protein in our model. And we had not perform the red reporters protein in the wet experiments. |
==''Reference''== | ==''Reference''== | ||
Revision as of 18:51, 3 October 2013
Contents |
FNR oxygen sensor system
As we use Hsim for the modeling, if you want to dig in our model we suggest you to read this page written by Mr. Patrick Amar the developer of Hsim.
Roughly speaking, we define a sampling volume, size in nanometer and we declare some molecules with their sizes, their moving speed and their number. Once the simulation starts, those molecules move randomly in the space and trigger some possible reaction between them with some probabilities. There is always somehow deviation from the reality, but we are all excited about this particular simulating modeling.
We represent our FNR regulator model to you by 3 steps. We have chosen HSIM, a multi-agents programme developped at our university in order to follow the assembly, movements and dissociation of a large number of molecules in a virtual cell. HSIM replaces the Ordinary Differential Equations and mimics chemical reactions of the real system by rewriting rules. Following our modeling process, we provided the list of molecules, the rewriting rules, and the initial values of each molecule quantity in a configuration file to HSIM. We also have developed a web version of Hsim which allows user change and test the system parameters.
Translate biological knowledges into functional scheme
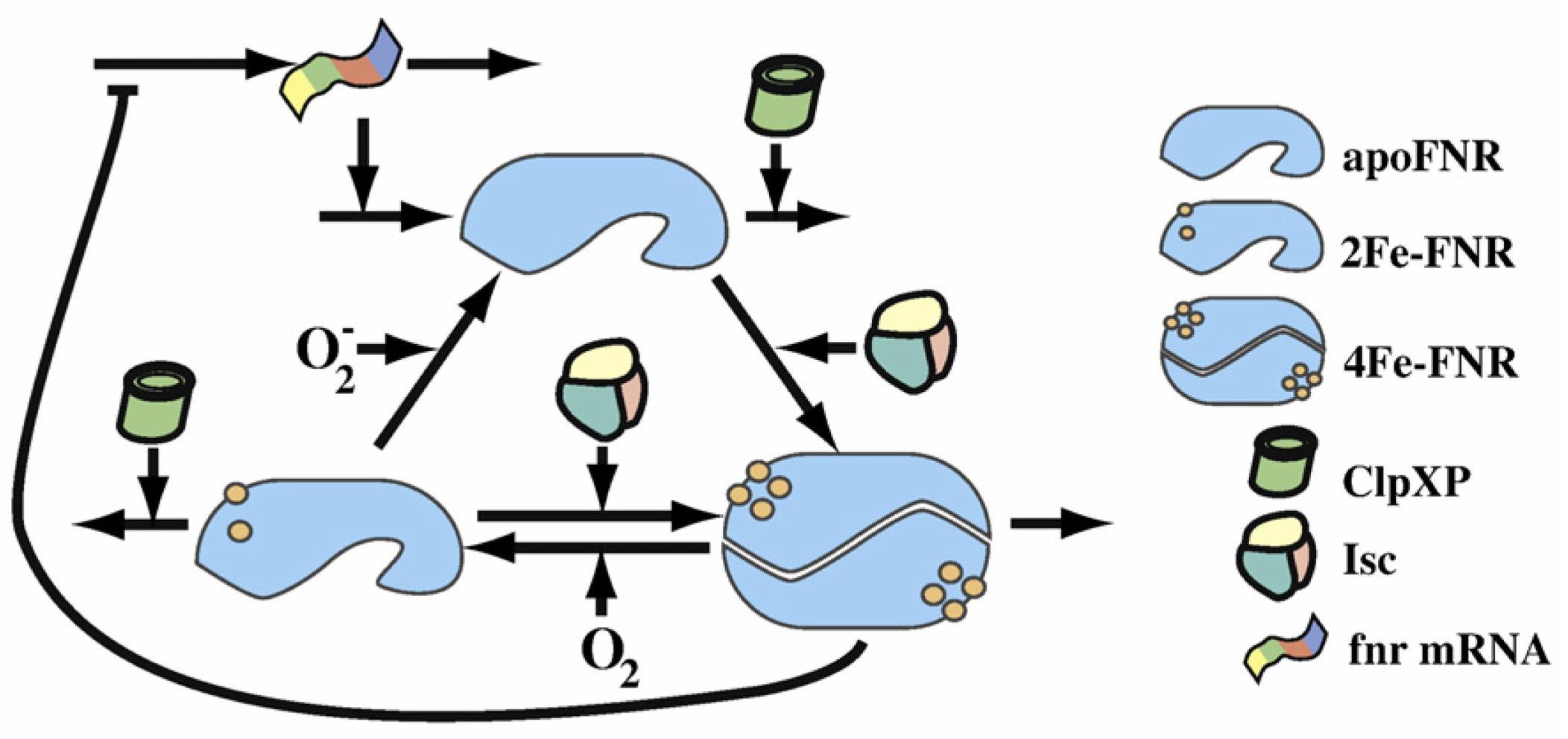
This scheme from the Dean A.Tolla and Michael A.Savageauthe's paper shows clearly the FNR transformation cycle. After the fnr mRNA translation, apoFNR is produced and a dimerization of 2 apoFNR catalyzed by ClpXP forme create the 4Fe-FNR. The 4Fe-FNR can be oxidized by oxygen to 2Fe-FNR then to apoFNR. 2Fe-FNR plus ClpXP can generate 4Fe-FNR. And all 3 FNR derivatives sustain a degradation effect. Those proteins will dissimilate in a while.
If we combine the FNR cycle and color reporter system :
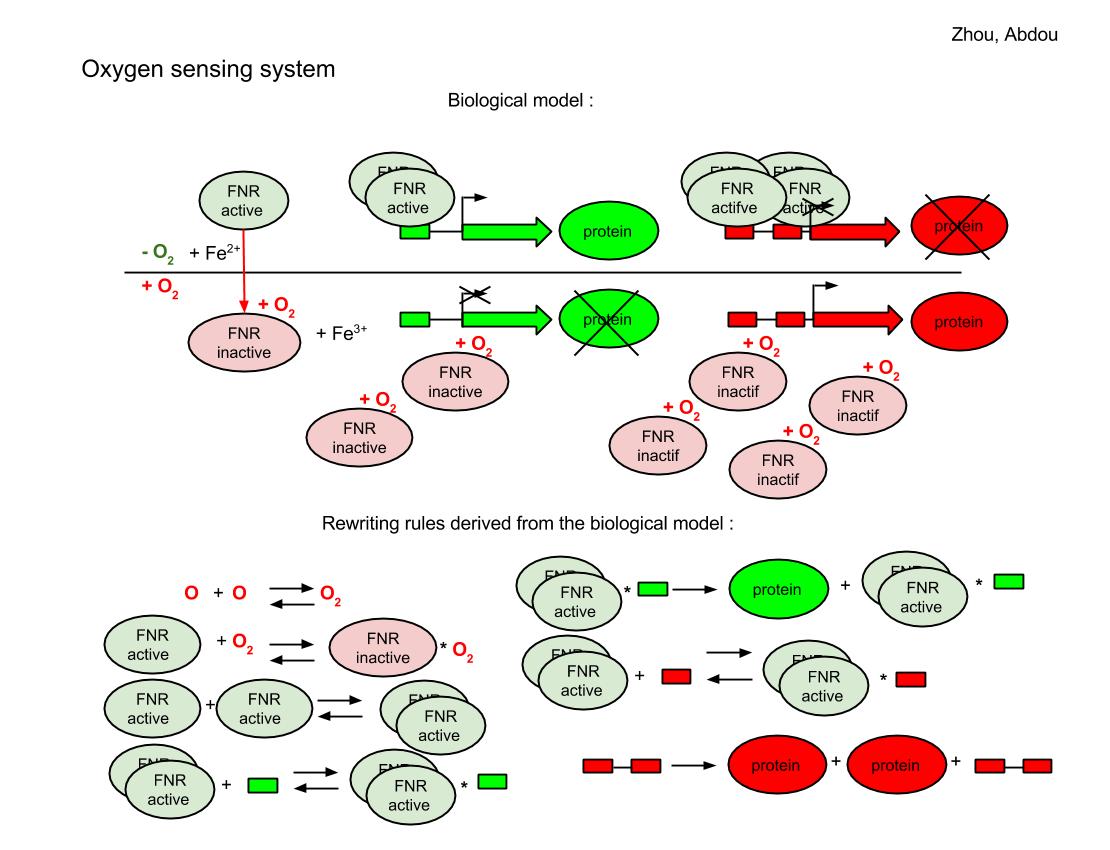
The FNR behave differently for aerobic and anaerobic condition. In the case that oxygen is absent, a pair of 4Fe-FNR fix on the reporter DNA sequence and the green reporter gene is expressed so we will see the green color for those colonies. But a group of 4 4Fe-FNR comes to fix at red reporter’s gene and prevents the expression of red color. If oxygen is sufficient, FNR derivatives keep in inactive form, the red reporter’s gene continue to produce the red color.
Transcribe functional description into equation system
Summary for equations:
- translation of Pfnr gene into FNR protein (FNR without Fe)
- dimerisation of 4Fe-FNR (action of Ise protein)
- oxydation: in aerobic conditions, oxygen inactivates FNR but cell continues to reactivate it (action of Ise protein)
- activation/inactivation of a gene under a repressor promotor (green) and production or not of the cognate protein
- activation/inactivation of a gene under an activator promotor (red) and production or not of the cognate protein
Details
- Translating fnr to FNR
- fnr mRNA generates steadily apoFNR(inactive).
- fnr -> fnr + apoFNR
- Dimerization of apoFNR
- 2 apoFNRs form active 4Fe-FNR(QFeFNRa) with catalyzer Ise.
- apoFNR + apoFNR + Ise -> QFeFNRa + Ise
- Oxidation, from 4Fe-FNR to apoFNR(inactive)
- In aerobical condition, oxygen inactivates FNR, from 4Fe-FNR to 2 inactive Fe-FNR(DFeFNRi)
- QFeFNRa + o2 -> DFeFNRi +o2
- Then, oxidize from 2Fe-FNR to apoFNR
- DFeFNRi + o2 -> apoFNR +o2
- 2Fe-FNR can be reactivate by Ise
- DFeFNRi + DFeFNRi + Ise -> QFeFNRa + Ise
- Degradation FNR
- Protein FNR and its derivation submit a steady degrading rate
- apoFNR + ClpXP -> ClpXP
- DFeFNRi + ClpXP -> ClpXP
- QFeFNRa + ClpXP -> ClpXP
- Activation/inactivation of green gene
- Association between 4Fe-FNR(activated) and green protein gene
- QFeFNRa + GPV -> QFeFNRa_GPV_binded
- Dissociation between 4Fe-FNR(activated) and green protein gene
- QFeFNRa_GPV_binded -> QFeFNRa + GPV
- QFeFNRa_GPV_binded -> PV + QFeFNRa_GPV_binded
- Green protein degradation :catalyzed by ClpXP
- PV + ClpXP -> ClpXP
- Repression of red protein gene : Association between 4Fe-FNR(activated) and red protein gene
- QFeFNRa + GPR -> QFeFNRa_GPR_binded
- Dissociation between 4Fe-FNR(activated) and red protein gene
- QFeFNRa_GPR_binded -> QFeFNRa + GPR
- Association of 4 4Fe-FNR(activated) binded, prevent the production of red reporter protein
- QFeFNRa_GPR_binded + QFeFNRa_GPR_binded -> repression_PR
- Dissociation of 4 4Fe-FNR(activated)
- repression_PR -> QFeFNRa_GPR_binded + QFeFNRa_GPR_binded
- Expression the red gene in aerobic condition
- Production of red protein without repression
- GPR -> GPR + PR
- QFeFNRa_GPR_binded -> QFeFNRa_GPR_binded + PR
- Degradation of red protein
- PR + ClpXP -> ClpXP
Scenarios and results
Here we prepared 3 scenarios in order to testing our model. Each of them represent one specific condition which can accord with our experiments. In the beginning of each scenario, we let our system run in the distribution mode for few generation, so molecules will spread homogeneously in the virtual bacterium.
- I. Degradation of red reporter protein
In this first scenario, we have a situation that the system has been in a anaerobic condition for a while, there is already some of red protein molecules. What we wish to see is a decline of red protein from initial value to 0, and it is what we observed in our model. There is also a latency due to the time needed for the formation of binding FNR molecules. The synthesize of green protein begins before oxygen running out.
[[]]
- II. Alternation of reporter protein
In this scenario, we changed the nature of oxygen from “metabolite” to “molecule” which means the quantity of oxygen in our system is no more constant, and distribution of oxygen become heterogeneous. We begin with 5000 molecules of oxygen, and 0 reporter protein. The red reporter protein is synthesized immediately. Oxygen is used to oxidant the FNR active to inactive state, however, when all oxygen is consumed, FNR n-mer begin to synthesize green protein and prevent red one. So we observe a exchange of curve position in the scheme. This scenario reveal the result of our experiments.
[[]]
Here you can find our configuration files for HSIM: link.
- III. Weird situation test
This scenario is a little bizarre, we set the presence of oxygen and a lot of green reporter at the same time. Our purpose is to test our model in some weird conditions, try to find some bug when the model reach its limit. And as we can see, after a period of latency, the system has found its way back to the normal situation.
[[]]
Online simulation
Simple simulation
Complex simulation
Conclusion
Our model reveal successfully the different states of FNR regulation. As you can see, in a anaerobic condition, the number of FNR active is significantly raised, so do the number of green reporter protein. In aerobic condition, because of the presence of oxygen, FNR inactive dominates in the model, the production of green ceases so the number of green protein drops, at the same time the repression for the red reporter disappear so production of red protein restart.
Back to our wet experiments, we used LacZ (blue color BBa_K11550007) and AmilCP (pink color BBa_K11550003) as 2 independent expression reporters which correspond to green reporter protein in our model. And we had not perform the red reporters protein in the wet experiments.
Reference
- Wenmao Meng, Jeffrey Green and John R.Guest FNR-dependent repression of ndh gene expression requires two upstream FNR-binding sites Microbiology (1997), 143, 1521-1532
- Parick Amar, Gilles Bernot, Victor Norris HSIM: a simulation programme to study large assemblies of proteins
Article written by Zhou and Damir
 "
"