Team:TU-Munich/Project/Biodegradation
From 2013.igem.org
(→Bielefeld 2012 Solution: BPUL Laccase) |
(→Active site and catalyzed reaction) |
||
| Line 152: | Line 152: | ||
====Active site and catalyzed reaction==== | ====Active site and catalyzed reaction==== | ||
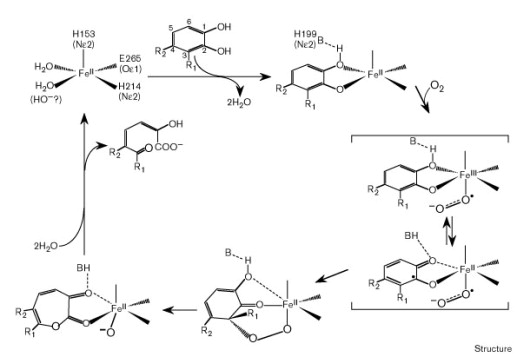
| - | [[File:Laccase_Copperoxidase.gif|thumb|right|435px|Figure 11]] | + | [[File:Laccase_Copperoxidase.gif|thumb|right|435px|'''Figure 11:''' Model of the active site of the Laccase as a Metalloprotease.]] |
The specific blue copper ions in the active site of Laccases are essential for the enzyme mediated radical oxidation of phenolic groups. In general, Laccases catalyze the linked oxidation of many aromatic and phenolic substances while the phenolic group is oxidized to a radical and oxygen is reduced to a hydrate. | The specific blue copper ions in the active site of Laccases are essential for the enzyme mediated radical oxidation of phenolic groups. In general, Laccases catalyze the linked oxidation of many aromatic and phenolic substances while the phenolic group is oxidized to a radical and oxygen is reduced to a hydrate. | ||
Revision as of 01:31, 5 October 2013
BioDegradation of Xenobiotics
Biodegradation is defined as "a process by which microbial organisms transform or alter the structure of chemicals introduced into the environment" [http://www.epa.gov/oust/cat/tumgloss.htm (U.S. Environmental Protection Agency, 2009)]. We work with this concept by degrading or transforming noxious substances in waste water into non-hazardous compounds. These substances, such as antibiotics, hormones or pesticides cannot be removed by common waste water treatment plants, but it is possible to inactivate them with existing enzymes from natural catabolic mechanisms in different microorganisms. To utilize these enzymes we integrated them into a plant as a self-sustaining sedentary organism to create a functional water filter system. To illustrate this approach we chose three potent enzymes:
- The Erythromycin esterase, which degrades macrolides, a persistant group of antibiotics
- The Laccase BPUL, which degrades several noxious substances, for example the artificial hormone ethinyl estradiol, the main ingredient of contraception pills
- The Catechol-2,3-dioxygenase, catalyzing the degradation of aromatic pollutants, which occur in pesticides and insecticides
Erythromycin Esterase (EreB)
The Problem: Antibiotics in the environment
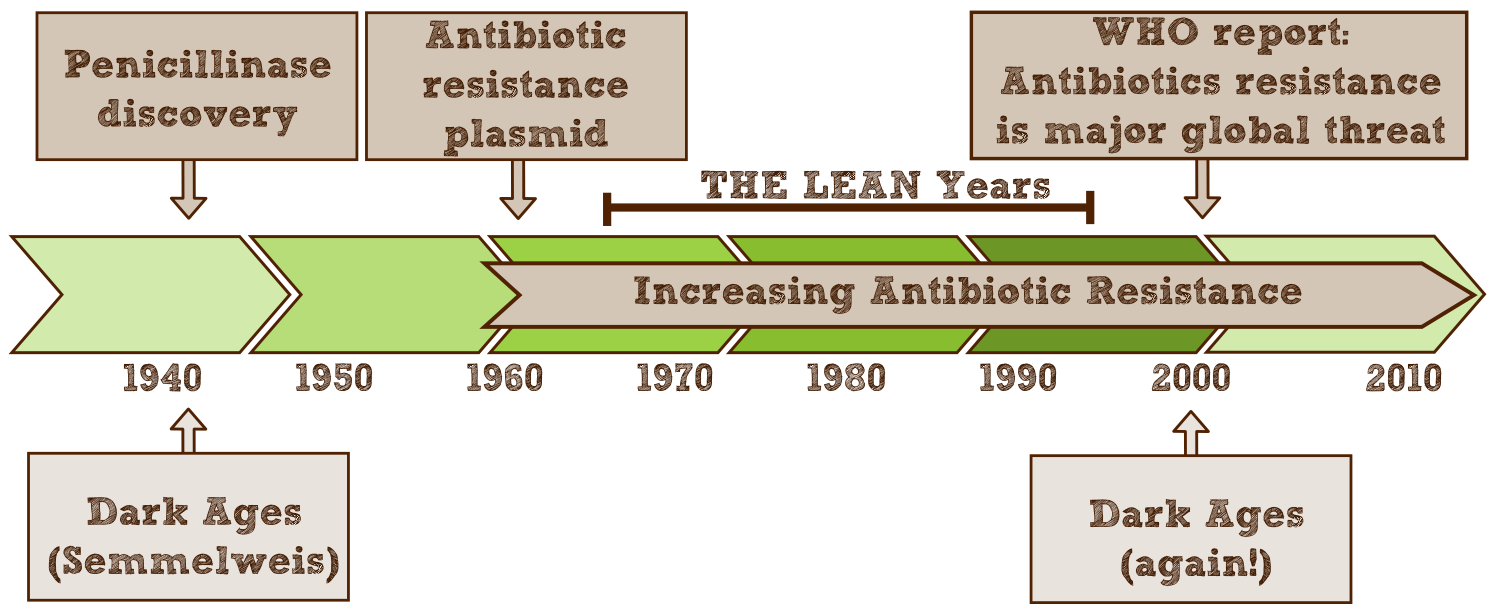
The discovery of antibiotics in the early 20th century has revolutionized the fight against infectious diseases. Due to their success thousands of tons of antibiotics have been produced, consumed and excreted, since antibiotics are designed to circulate through the body for a long time and therefore up to 75% leave the organism unaltered. As a result microbes are exposed to strong selective pressure to which these numerous and extremely adaptable organisms react by developing resistance mechanisms.
However, antimicrobial effectiveness is a precious, limited resource, that we can no longer do without. Accordingly, in 2000 a [http://www.who.int/infectious-disease-report/2000/ World Health Organization report] focused on antibiotic resistance as one of the most critical human health challenges of the next century and called for “a global strategy to contain resistance”. In numbers, the WHO estimates that resistant pathogens affect 2 million Americans each year, of which 14,000 die as a result. In the EU antimicrobial resistance is responsible for 25,000 deaths every year and the annual treatment and social costs have been estimated at some €1.5 billion http://www.ecdc.europa.eu/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf ECDC/EMEA joint technical report 2009. These numbers will only rise if nothing is done to prevent precious antibiotics from becoming effectless. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2937522/ Davies and Davies, 2010
Current Solutions
The obvious approach to minimize selective pressure on microorganisms is to deal with the problem of carefree, often precautionary overuse of antibiotics, not only in humans but also and especially in animal breeding, by only using them when absolutely necessary.
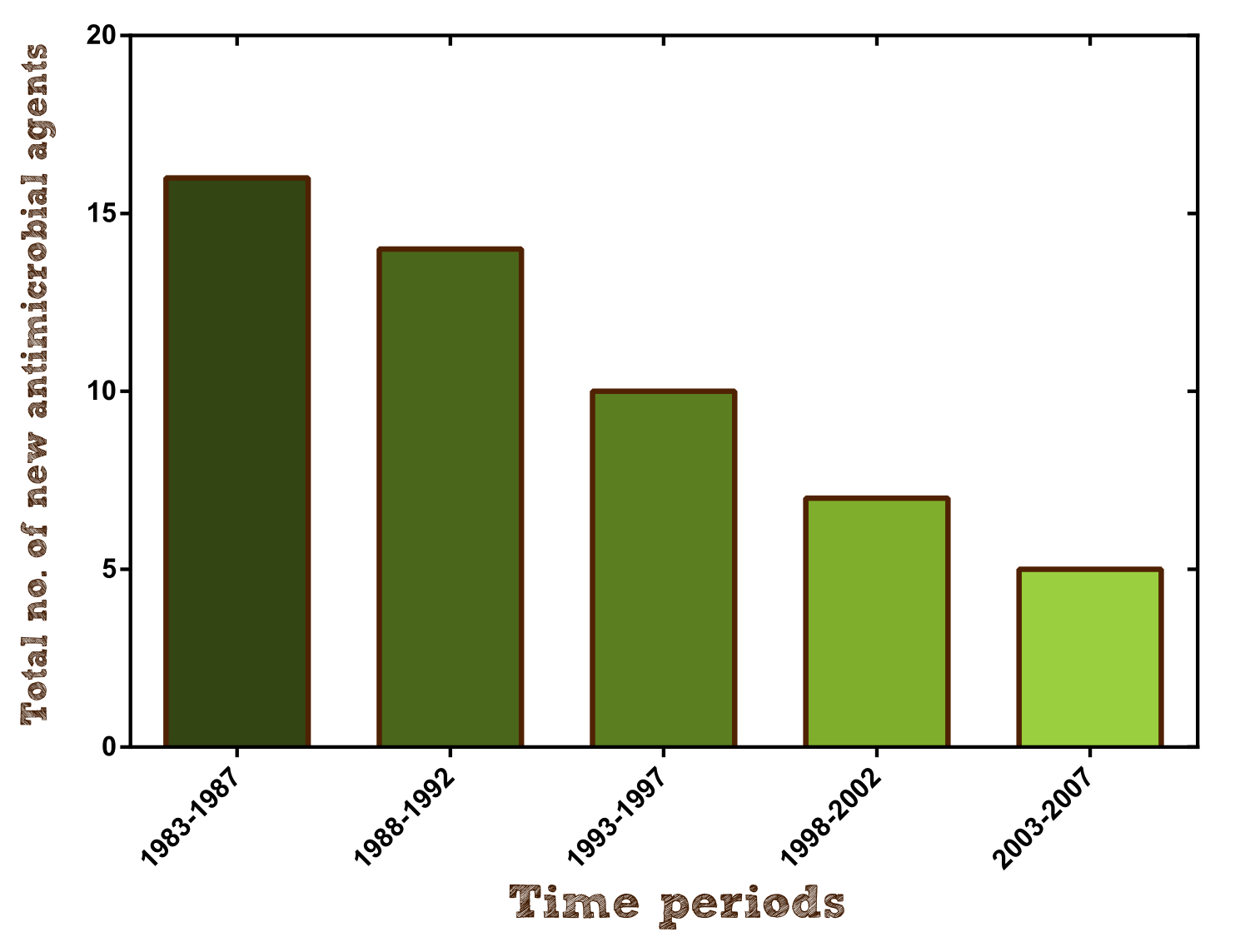
Furthermore research on new antibiotics should be hugely intensified to create alternative agents and keep pace with the formation of antimicrobial resistance. Unfortunately antibiotic development has slowed dramatically over the past 25 years because as a short-course and very effective therapy they have a much lower rate of return on investment than other drugs and therefore are financially not viable for pharmaceutical companies (per approved agent development costs are estimated to be $400-$800 million). For example in 2004 only 5 new antibacterial agents were under development by the largest pharmaceutical companies, barely beating the 4 new molecular entities to treat erectile dysfunction. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2937522/ Davies and Davies, 2010
These two options are difficult to accomplish due to the increasing need of antibiotics with a growing world-wide population and the fact that finding new antibiotics is a costly, long-term and extremely difficult project. Therefore we focus on a third option to fight the spread of antibiotic resistance by developing a cheap, innovative water-filter that will prevent continuous inflow of the drugs into surface water and therefore reduce the selective pressure on microbes.
Our Solution: EreB
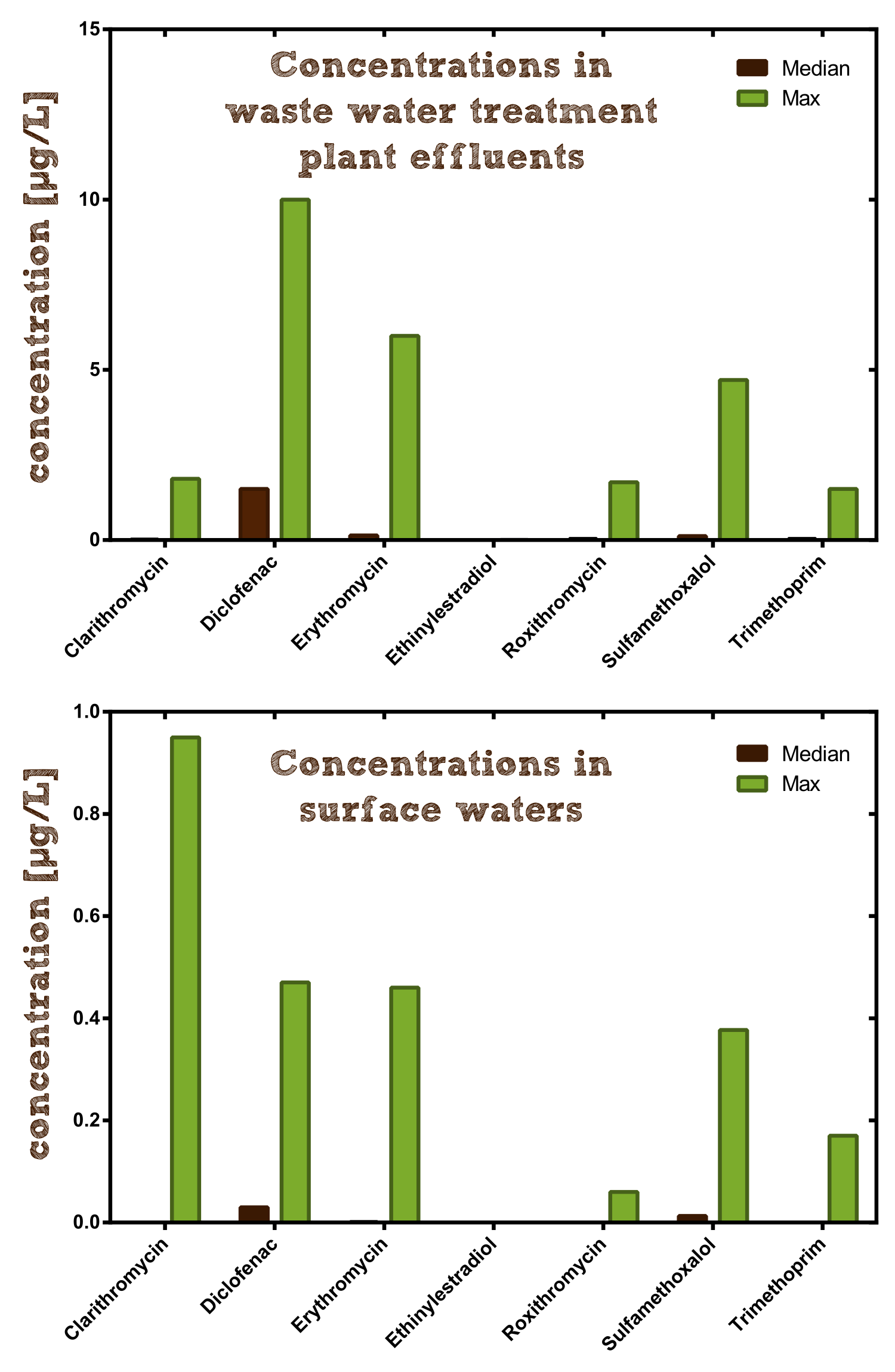
In our search for a suitable enzyme we looked at antibiotic concentrations in waste water. There have not yet been many investigations on this topic, one of the main surveys was executed by the [http://www.blac.de/servlet/is/2146/P-2c.pdf German national committee for chemical safety (BLAC)] of 202 effluent samples from 34 waste water treatment plants (WWTP). The result was that the main detectable antibiotics are the macrolides Erythromycin, Roxythromycin and Clarithromycin, the Sulfonamide Sulfamethoxalol as well as the Fluoroquinolone Ciprofloxacin. Most concentrations are about 100 ng/l, but maximum concentrations reached more than 3 µg/l. The often prescribed ß-lactam antibiotics, such as penicillin, were not detectable probably due to their fast degradation. Another important conclusion of the study is that the main source of antibiotic input into the aquatic environment is municipal waste water. Similar results were found in other countries (see Table 1).
| Antibiotic | max. concentration [µg/l] found in different countries[http://www.umweltbundesamt.at/fileadmin/site/publikationen/REP0258.pdf [4]] | min MIC50[http://antibiotics.toku-e.com/a/[6]][µg/l] |
|---|---|---|
| Ciprofloxacin (fluoroquinolone) | 0.14 (D), 0.18 (USA), 0.74 (N) | 3 |
| Clarithromycin (macrolide) | 1.00 (D), 0.30 (CH) | 2 |
| Erythromycin (macrolide) | 1.10 (D), 0.17 (USA), 0.29 (CH), 2 (China) | 4 |
| Roxythromycin (macrolide) | 1.00 (D), 0.28 (China) | 15 |
| Sulfamethoxalol (sulfonamide) | 4.00 (D), 1.40 (USA) | 60 |
| Trimethoprim (diaminopyrimidine) | 1.50 (D), 1.20 (USA), 1.30 (N) | 120 |
Now we compared the concentrations of the most frequent antibiotics found in WWTP effluents to the [http://en.wikipedia.org/wiki/Minimum_inhibitory_concentration minimum inhibitory concentration] (MIC50) at which the growth of at least 50% of bacterial strains are inhibited. The MIC50 depends on the species. In the table the smallest MIC50 over all species is given, indicating the level at which the contamination seriously affects the microbiological system and therefore carrying out a certain stress which gives resistant individuals an increased selective advantage.
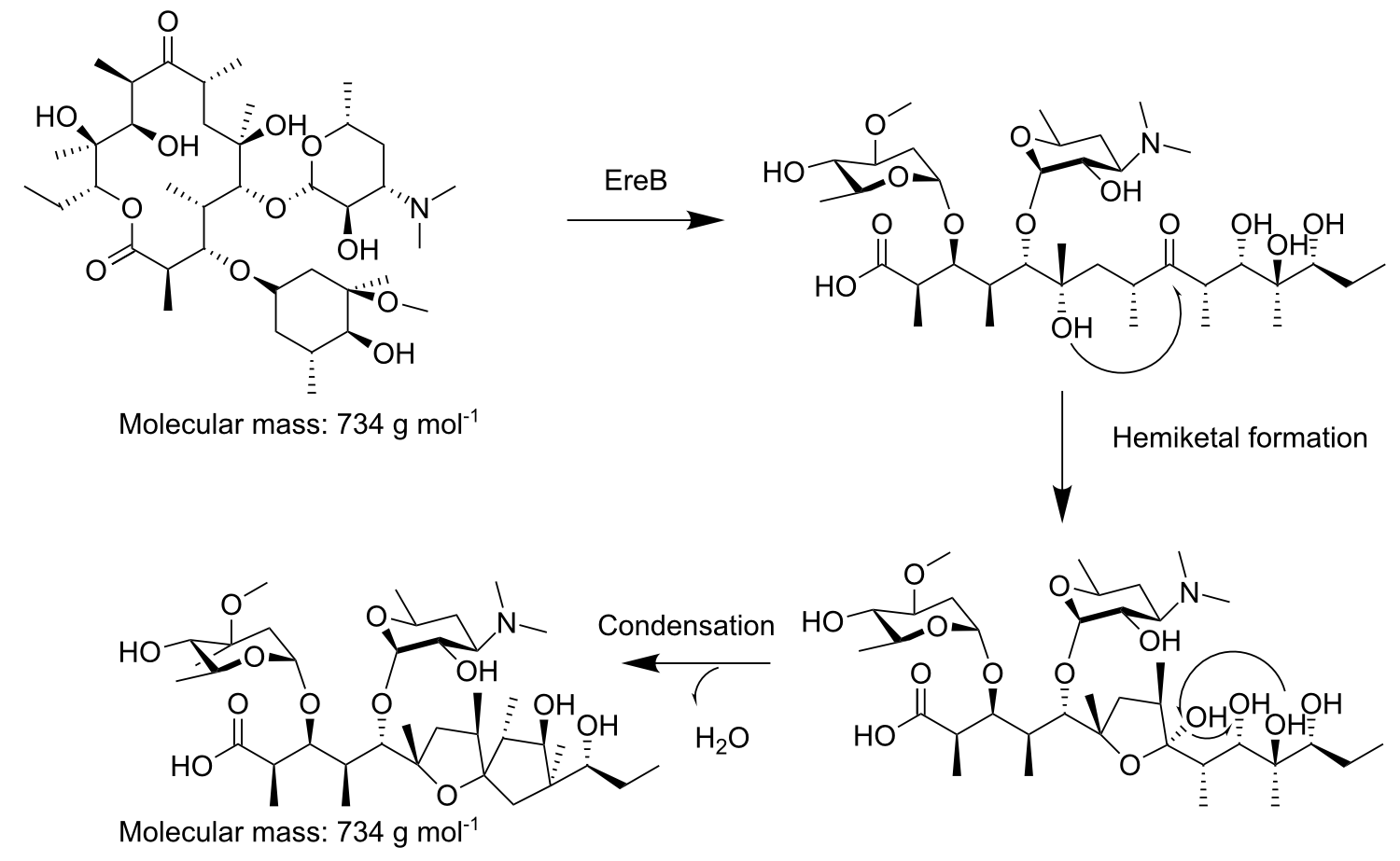
Our conclusion from these data was that macrolides are a hazardous antibiotic class, from which several representatives are found in WWTP effluents and surface water in concentrations relatively close to their MIC50. Macrolides are important and clinically relevant agents used to treat infectious diseases, first introduced into clinics in 1952 as an alternative to penicillin. Today they are mainly applied for treatment of respiratory diseases, including pneumonia, the leading cause of childhood deaths worldwide. The drug functions by inhibiting protein biosynthesis when it binds to the exit tunnel of the 50S ribosomal subunit which leads to abortion of the growth of nascent peptide chains. Macrolides can be enzymatically inactivated with the macrolide esterases EreA and EreB, from which we chose EreB as our model enzyme since it has a broader degradation spectrum than EreA and is not metal-dependent.
EreB is a cytoplasmic protein, originally discovered in E. coli on an exogeneous plasmid, that belongs to the hydrolase superfamily catalyzing the opening of the macrolactone ring, that is characteristic for macrolides. After the enzymatic opening of this cyclic ester bond a non-enzymatic hemiketal formation takes place followed by condensation and dehydration. The only co-substrate needed by the enzyme for hydrolysis is water which also makes it a suitable candidate for our moss-filter.
More information on the enzyme can be found for example in a very detailed recently published paper by http://pubs.acs.org/doi/abs/10.1021/bi201790u?mi=0&af=R&pr... Wright et al., 2012, [http://130.113.155.235/thewrightlab/ who's group] also generously provided us with the plasmid DNA.
Catechol Dioxygenase (xylE)
Catechol: A growing concern
As ever more third world countries around the globe experience a rapid industrialization without establishing proper environmental practices as well as big companies outsourcing their production facilities in order to avoid expensive sewage treatment the problem of water pollution is more urgent then ever. The Ministry of Environment and Forests, Government of India and EPA, USA have listed Phenol and phenolic compounds as priority pollutants http://www.neeri.res.in/project_details.php?PID=261&DIV=38 Ministry of Environment and Forest http://water.epa.gov/scitech/swguidance/standards/criteria/health/phenol_index.cfm EPA. In this context, a derivative of benzene, the phenolic compund catechol, or 1,2-dihydroxybenzene, has been deemed to be a very toxic pollutant http://www.epa.gov/ttnatw01/hlthef/pyrocate.html Informations about Catechol by the EPA. According to the U.S. National Library of Medicine´s Hazardous Substances Data Bank (HSDB) the widespread use of catechol in pharmaceutical and agricultural products as well as in the rubber, chemical, photographic, dye and oil industries poses a risk of water contamination through various waste water streams http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+1436 HSDB about Catechol.
| Organism group | Observed effects of catechol on |
|---|---|
| AquaticPlants | Biochemistry, Cell(s), Population |
| Crustaceans | Mortality |
| Fish | Behavior, Biochemistry, Enzyme(s), Hormone(s), Mortality, No Effect Coded, Physiology |
| Phytoplankton | Behavior, Biochemistry, Cell(s), Intoxication, Mortality, Physiology, Population |
| Zooplankton | Feeding Behavior, Mortality, Population |
Due to its physical properties and the localization of its artificial contamination effects of catechol are most severe in aquatic environments [ref]. This allows our water based moss filter to target the problem at its roots. It is shown that catechol pollution has dramatic consequences for aquatic life ranging from disturbances in an organisms biochemistry all the way to anomalies in whole populations.
Current Solutions
Today catechol can be treated by a variety of methods including treatment by bioregenerated anaerobic granular activated carbon (GAC), anaerobic biological treatment using upflow anaerobic fixed-film fixed-bed reactors as well as upflow anaerobic sludge blanket (UASB) reactors, with the latter being the most promising one. Despite their decent efficiency these methods lack the applicability where they are most needed, rural areas in third world countries most affected by the pollution of the surrounding oil and gas industries. The aforementioned technologies all need skilled technicians, a constant supply of water and electricity and of course money to maintain them. These are all factors that got us to look deeper into a simpler and more cost-effective way to address those problems: Phytoremediation.
Our Solution: A new approach with old methods
Further research into this topic showed that catechol was already a popular target for phytoremediation were it was degraded by Arabidopsis thaliana http://www.ncbi.nlm.nih.gov/pubmed/16935988 Liao et al., 2006. After already establishing a whole new chassis and a new effector in our project we decided that we would tackle at least one problem in the original spirit of iGEM, that is taking an existing part and implement it into your own system. To do this we selected an RFC25 - ready brick [http://parts.igem.org/Part:BBa_K648011 BBa_K648011], which was originally designed as reporter construct, and implemented it in our moss filter.
Catecholdioxygenase
The enzyme we used to degrade catechol is catechol-2,3-dioxygenase, or metapyrocatechase, from Pseudomonas putida. This enzyme is a homotetramer with a non heme ferrous ion at each subunit´s active site. It belongs to the class of oxidoreductases and catalyzes an extradiol cleavage of catechol, forming the brown 2-hydroxymuconate semialdehyde which can be detected photometrically at wavelenghts of around 380-400 nm and can therefore act as a reporter of the reaction.
Goals
By doing this we hope to eliminate many existing problems with the practical application of catecholdegredation when using bacteria as a chassis. Namely the facts that bacteria, other than plants, are neither self-sustaining nor easy to handle for non-experts. Combined with our red-light inducible kill-switch we wanted to create a system which could be used nearly everywhere and by anyone and be as cheap as possible. This way we could ensure that our system would be where it is most needed and would be affordable for every community.
Laccase (Bacillus pumilus)
The Problem: Endocrine Disruptors in the Environment
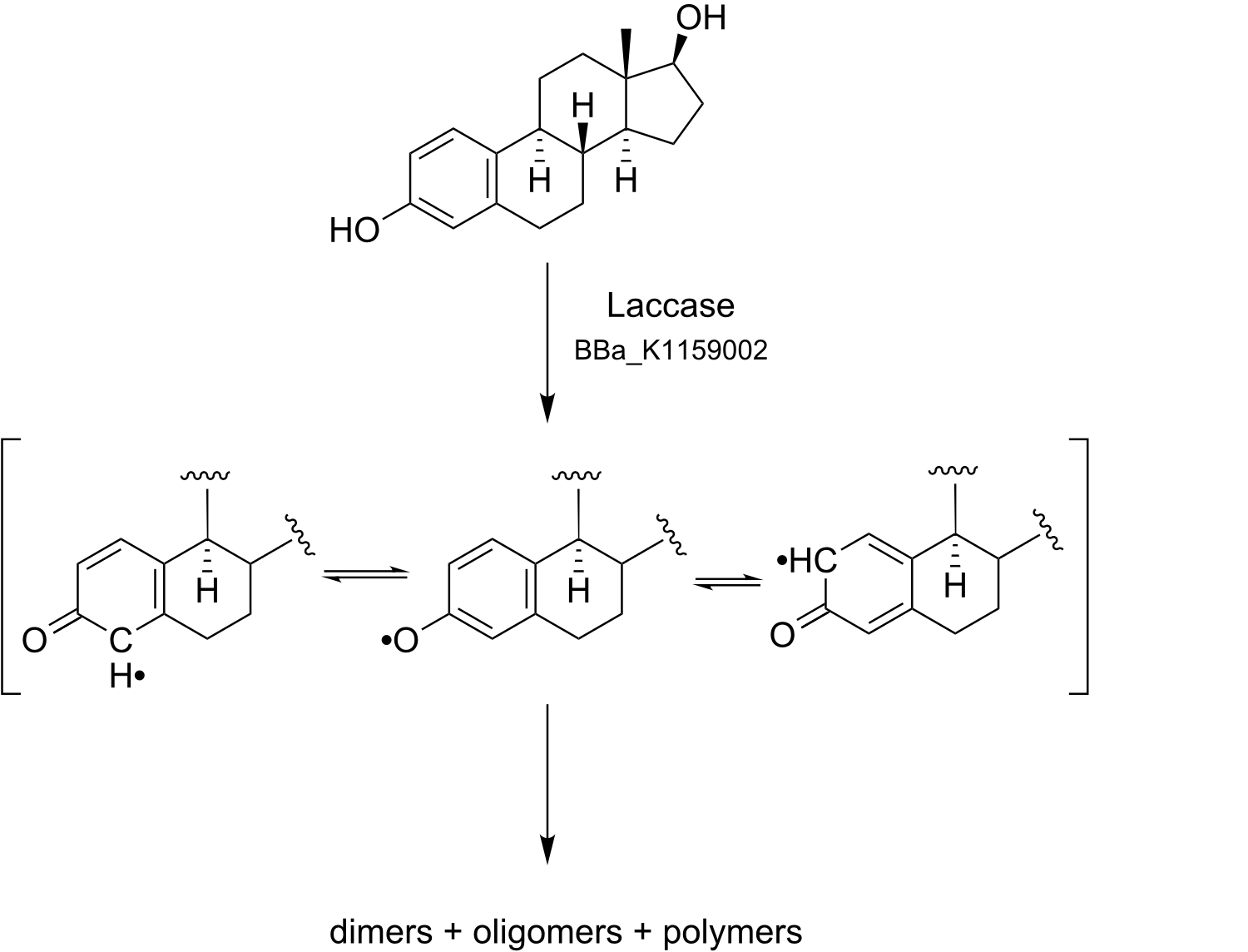
The concentration of Estrogens in ground water rises steadily http://www.ingentaconnect.com/content/wef/wefproc/2005/00002005/00000005/art00020 Okayasu et al. (2005), because of the increasing receipt of contraceptics based on synthetic estrogens. Ground water, cleaned by standard sewage plants, is still contaminated with microelements like Estrogens and painkillers like Diclofenac in low concentrations, causing severe endocrine disorders in animals and humans. Known consequences - until now - are for example reduced fertility and feminization of animals living in an aquatic environment. Further on, Estrogens belong to the chemical class of Polycyclic aromatic hydrocarbons, which are charaterized by a low biodegradability and a high bioaccumulation rate.
Current Solutions: Ozonation, Nitrification and Denitrification
Possible solutions to degrade Estradiol are widely spread and very ineffective until now. Researchers are continuously trying to find an effective way to degrade or biodegrade this chemical compound for example by using Ozonation, Nitrification and Denitrification.
Using and comparing Nitrification (aerobic conditions) and Denitrification (anaerobic conditions) http://www.ncbi.nlm.nih.gov/pubmed/18254398 Dytczak et al. (2008) the researchers concluded that denitrification did not work either but the nitrification rate seemed to be at 22%. The Nitrification rate correlated with the amount of used biomass.
Further on the was described, where Estradiol was degraded by varying the concentration of Ozon and the pH value http://www.ncbi.nlm.nih.gov/pubmed/16387433 Zhang et al. (2006). As described in the paper by Zhang et al., small molecules appeared as byproducts, which have been responsible for a rapid shrinkage of the pH value. This means that byproducts during degradation are acidic, which may harm the environment in a significant way. Another experimental proven way of ozonating Estradiol is the use of Manganoxid and oxalic acid http://www.ncbi.nlm.nih.gov/pubmed/23530323 Jiang et al. (2013), which seems to be a promising alternative for degrading Estradiol nearly completely. Nevertheless this method will also cause accumulation of byproducts.
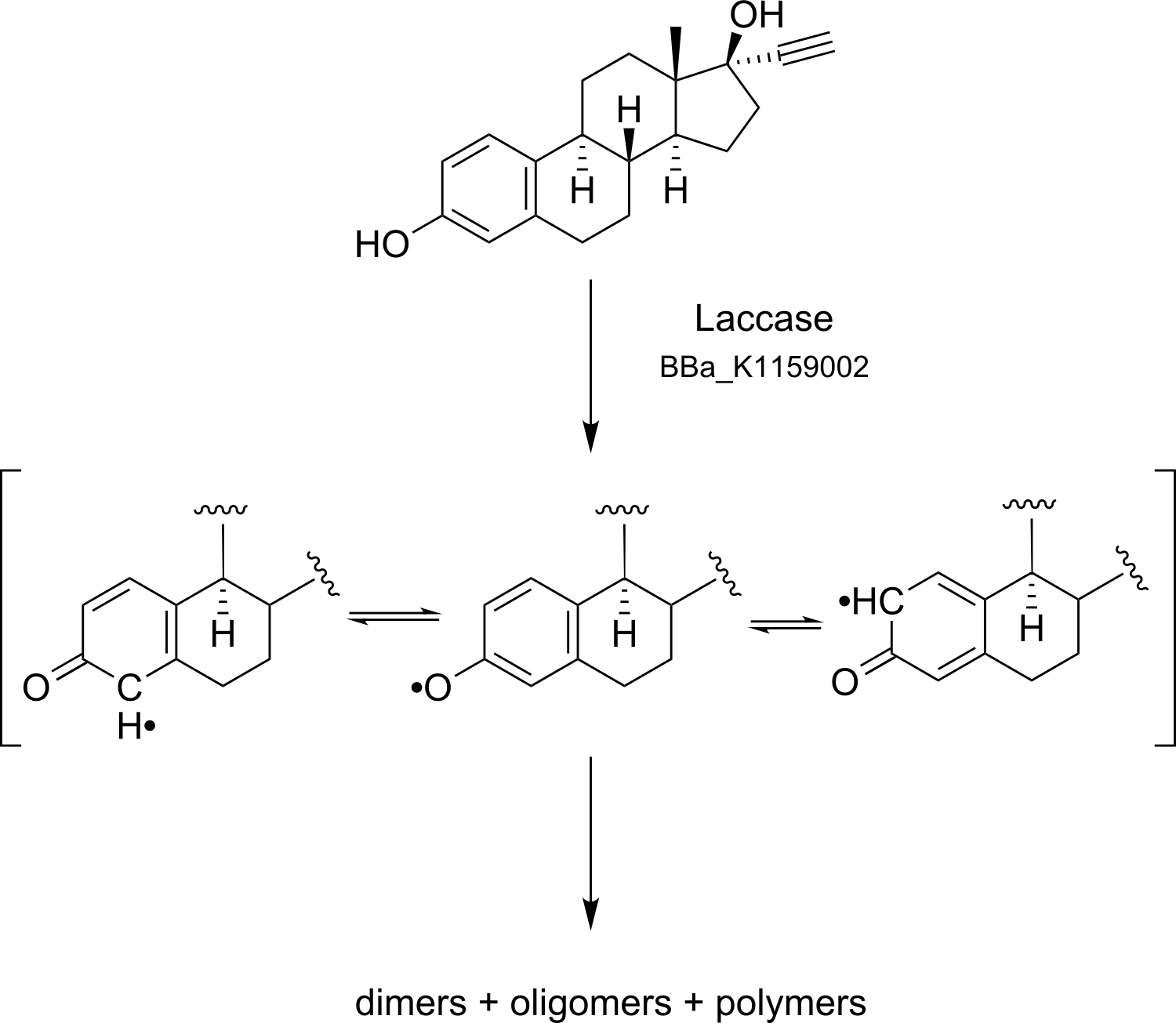
Bielefeld 2012 Solution: BPUL Laccase
All derivates of Estrogen are Polycyclic aromatic hydrocarbons, which are hard and non cost effective removable by standardized purification methods in sewage plants. One possible sollution to biodegrade these Polycyclic aromatic hydrocarbons envisioned by the 2012 iGEM Team from Bielefeld is the Laccase, which is a well characterized, widely used and solid protein while environmental conditions are varying. Its substrate spectrum promises a broad range of applications, especially biodegrading Estradiol, Estrone, Ethinyl estradiol, Diclofenac, Naphthalene and Anthracene.
General Information on Laccases
Laccases are implicated in a wide spectrum of biological activities and, in particular, play a key role in morphogenesis, development and lignin metabolism in fungi, bacteria and plants. For example, they are necessary to degrade lignin in Basidiomycetes and synthesize complex polymers like Melanin in Ascomycentes. Laccases belong to the [http://www.chem.qmul.ac.uk/iubmb/enzyme/ enzyme class of Oxidoreductases (EC 1.10.3.2)]. They are also known as monophenolic Oxidases, because of their substrate specifity. It is widely believed that Laccases are the simpliest representatives of the ubiquitous blue multi-copper oxidase family. Laccases are extracellular enzymes consiststing usually of 15-20 % carbon-hydrogen. Their molecular weight ranges deglycated from 60 to 80 kDa. Further on Laccases can occur as monomers, dimers, trimers and tetramers.
Active site and catalyzed reaction
The specific blue copper ions in the active site of Laccases are essential for the enzyme mediated radical oxidation of phenolic groups. In general, Laccases catalyze the linked oxidation of many aromatic and phenolic substances while the phenolic group is oxidized to a radical and oxygen is reduced to a hydrate. The active site of the enzyme includes a four-copper-ion-cluster which can be detected by using Spectroscopy. The cluster consists of one type 1 blue copper-ion, one type 2 and two type 3 copper-ions. In this reaction the electron from the oxidation is transfered to the other three copper ions. These ions form a trinuclearic cluster, which transfers electrons to the terminal electron acceptor oxygen. In the end, molecular oxygen is reduced, by receiving four electrons, to water.
Possible applications of Laccases
Due to their ability to oxidize phenolic and nonphenolic lignin related compounds, as well as highly recalcitrant environmental pollutants, Laccases possess a high potential as a bioremediation agent in synthetic biology. Waste water from hospitals, textile and paper industry, as well as in sewage treatment plants can be detoxified. Possible substrates may be natural and synthetic Estrogens, Xenoestrogens and polycylic aromatic hydrocarbons. Laccases may also be used to degrade high polymeric Lignin to make use of the polymer for energy generation. Carcinogenic, mutagenic aromatic hydrocarbons and estrogens, xenoestrogens and polycyclic aromatic hydrocarbons can be oxidized and detoxified by laccases, too. In addition to that, Laccases can oxidize up herbicides, pesticides in soil.
Laccase BPUL from Bacillus Pumilus [http://parts.igem.org/Part:BBa_K863000?title=Part:BBa_K863000 BBa_K863000]
In this project, we used the BPUL Laccase from Bacillus Pumilus [http://parts.igem.org/Part:BBa_K863000?title=Part:BBa_K863000 BBa_K863000], which was established and well characterized by the iGEM Team of Bielefeld in 2012 . The Laccase, which was prepared and sent to the Parts Registry by the team of Bielefeld was fused to a Histag and a Ribosome Binding Site. The molecular weight of this Laccase is 58.6 kDa. Comparing the enzyme activity between 25°C and 10°C, you can see that it is influenced marginal, but it slightly decreases. Its pH optimum is reached at pH 4 to 5. The differences in activity varying the concentration of CuCl2, differ minimal. An optimum is reached at 0.6 mM. An increase of the Concentration of CuCl2 decreases the enzyme activity of the Laccase. The lower the used concentration of MeOH and Acetonitrile was, the higher the enzyme activity was detected. Bielefeld established many different Laccases in iGEM 2012, but we chose this one, because it seemed to be the most effective one, they have characterized.
References:
- http://www.rsc.org/chemistryworld/2013/03/antibiotic-resistance-ticking-time-bomb Antibiotic resistance is a `ticking time bomb` Stafford, N. (2013). Antibiotic resistance is a `ticking time bomb`. Advancing the chemical sciences.
- http://www.who.int/infectious-disease-report/2000/ WHO report 2000 World Health Organization Report on Infectious Diseases (2000).
- http://www.ecdc.europa.eu/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf ECDC/EMEA joint technical report 2009 The bacterial challenge: time to react. ECDC/EMEA joint technical report (2009).
- http://www.umweltbundesamt.at/fileadmin/site/publikationen/REP0258.pdf report on antibiotics in ground water, 2010 Clara M., Gans O., Humer F., et al (2010). Antibiotics in ground water. Federal austrian environmental agency report. Vienna
- http://www.blac.de/servlet/is/2146/P-2c.pdf report on antibiotics in the .environment, 2003 Rohweder, U. (2003). Antibiotics in the environment. German national committee for chemical safety (BLAC).
- http://antibiotics.toku-e.com/a/ The antimicrobial index
- http://www.watereuse.org/files/images/91051.pdf Occurrence survey of pharmaceutically active compounds (2005) Sedlak, D., Pinkston, K., Huang, C. (2005). Occurrence survey of pharmaceutically active compounds. WateReuse foundation.
- http://faculty.tamucc.edu/plarkin/4292folder/Ab resistance - enzymatic.pdf Wright, 2005 Wright, G. (2005). Bacterial resistance to antibiotics: Enzymatic degradation and modification. Advanced Drug Delivery Reviews, 57: 1451–1470.
- http://pubs.acs.org/doi/abs/10.1021/bi201790u?mi=0&af=R&pr... Wright et al., 2012 Morar, M., Pengelly, K., Koteva, K. and Wright, D. (2012). Mechanism and Diversity of the Erythromycin Esterase Family of Enzymes. Biochemistry, 51(8): 1740–1751.
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2937522/ Davies and Davies, 2010 Davies, J. and Davies, D. (2010). Origins and Evolution of Antibiotic Resistance. Microbioly and Molecular Biology Reviews, 74(3): 417–433.
- http://cid.oxfordjournals.org/content/46/2/155.long Spellberg et al. 2008 Spellberg et al. (2008). The Epidemic of Antibiotic-Resistant Infections: A Call to Action for the Medical Community from the Infectious Diseases Society of America. Clinical Infectious Diseases, 46(2): 155-164.
- http://www.neeri.res.in/project_details.php?PID=261&DIV=38 Ministry of Environment and Forest
- http://water.epa.gov/scitech/swguidance/standards/criteria/health/phenol_index.cfm EPA about Phenolderivatives
- http://www.epa.gov/ttnatw01/hlthef/pyrocate.html EPA about Phenol
- http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+1436 HSDB about Catechol
- http://www.ncbi.nlm.nih.gov/pubmed/16935988 Liao et al., 2006 Liao Y, Zhou X, Yu J, Cao Y, Li X, Kuai B. (2006). The key role of chlorocatechol 1,2-dioxygenase in phytoremoval and degradation of catechol by transgenic Arabidopsis.
- http://www.ncbi.nlm.nih.gov/pubmed/12126701 Mayer AM, Staples RC, 2002 Mayer AM, Staples RC (2002). Laccase: new functions for an old enzyme. Phytochemistry, 60(6):551-65.
- http://www.ncbi.nlm.nih.gov/pubmed/15036303 Claus H.2004 Claus H. (2004). Laccases: structure, reactions, distribution. Micron., 2004;35(1-2):93-6.
- http://www.ncsu.edu/bioresources/BioRes_04/BioRes_04_4_1694_Madhavi_Lele_Laccase_Properties_Applications_Review_567.pdf Madhavi and Lele (2009) Madhavi and Lele (2009). Laccase properties, use. Bioresources, 4(4), 1694-1717.
- http://www.ingentaconnect.com/content/wef/wefproc/2005/00002005/00000005/art00020 Okayasu et al. (2005) Degradation of Free Estrogens and their Conjugates in Wastewater Treatment Process. Proceedings of the Water Environment Federation, Technology 2005 291-297(7)
- http://www.ncbi.nlm.nih.gov/pubmed/18254398 Dytczak MA, Londry KL, Oleszkiewicz JA (2008) Biotransformation of estrogens in nitrifying activated sludge under aerobic and alternating anoxic/aerobic conditions. Water Environ Res. 2008 Jan;80(1):47-52.
- http://www.ncbi.nlm.nih.gov/pubmed/16387433 Zhang et al. (2006) Degradation of 17-ethinylestradiol in aqueous solution by ozonation. Journal of Hazardous Materials 291–298
- http://www.ncbi.nlm.nih.gov/pubmed/23530323 Jiang et al. (2013) Degradation of 17beta-estradiol in aqueous solution by ozonation in the presence of manganese(II) and oxalic acid. Environ Technol. 2013 Jan-Feb;34(1-4):131-8.
 "
"





AutoAnnotator:
Follow us:
Address:
iGEM Team TU-Munich
Emil-Erlenmeyer-Forum 5
85354 Freising, Germany
Email: igem@wzw.tum.de
Phone: +49 8161 71-4351