Team:ZJU-China/Project/Safety/CaCO3Shell
From 2013.igem.org
m (→Characterization) |
(→Characterization) |
||
| Line 71: | Line 71: | ||
[[File:ZJU-CaP-9.jpg|200px]] | [[File:ZJU-CaP-9.jpg|200px]] | ||
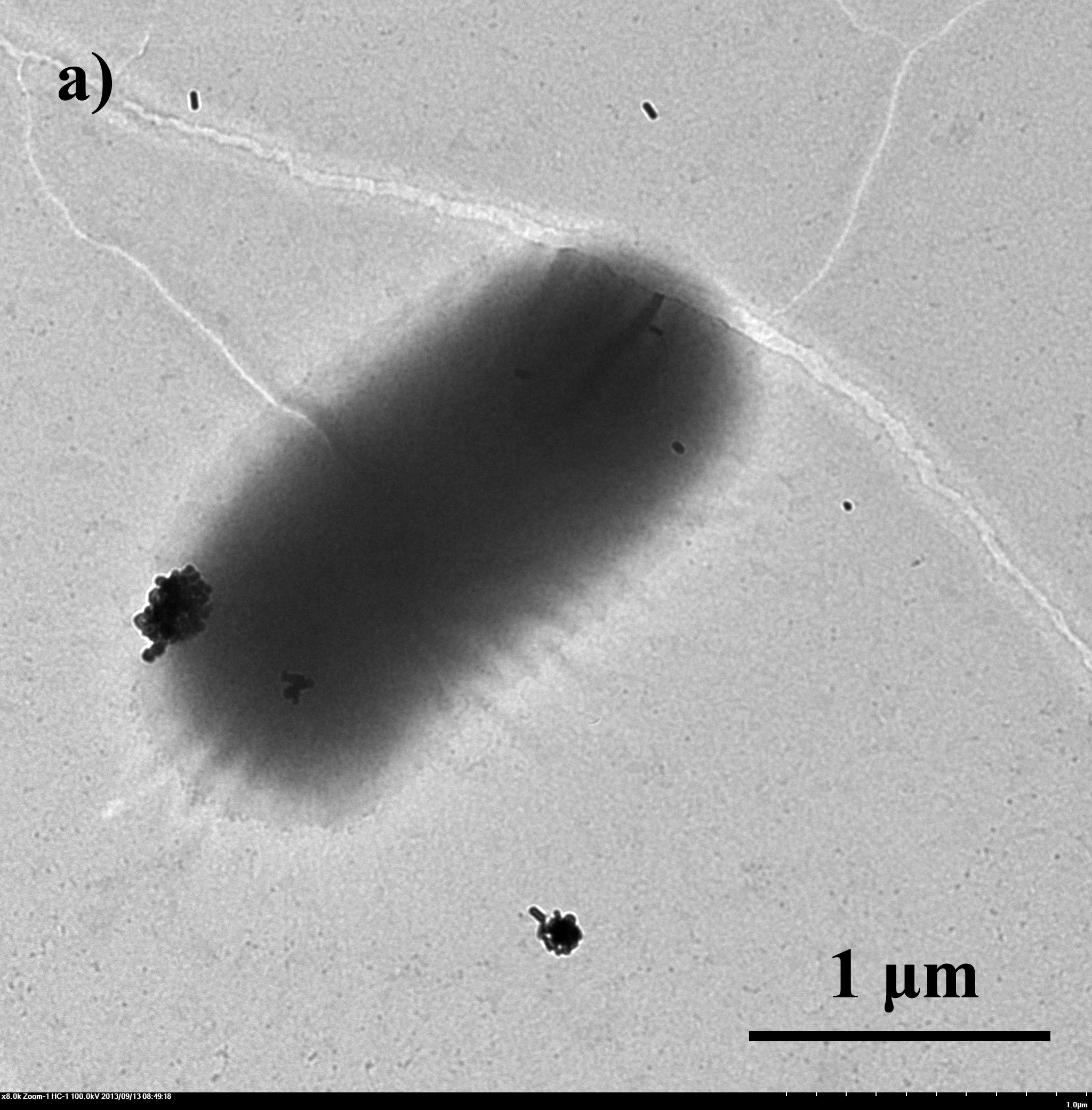
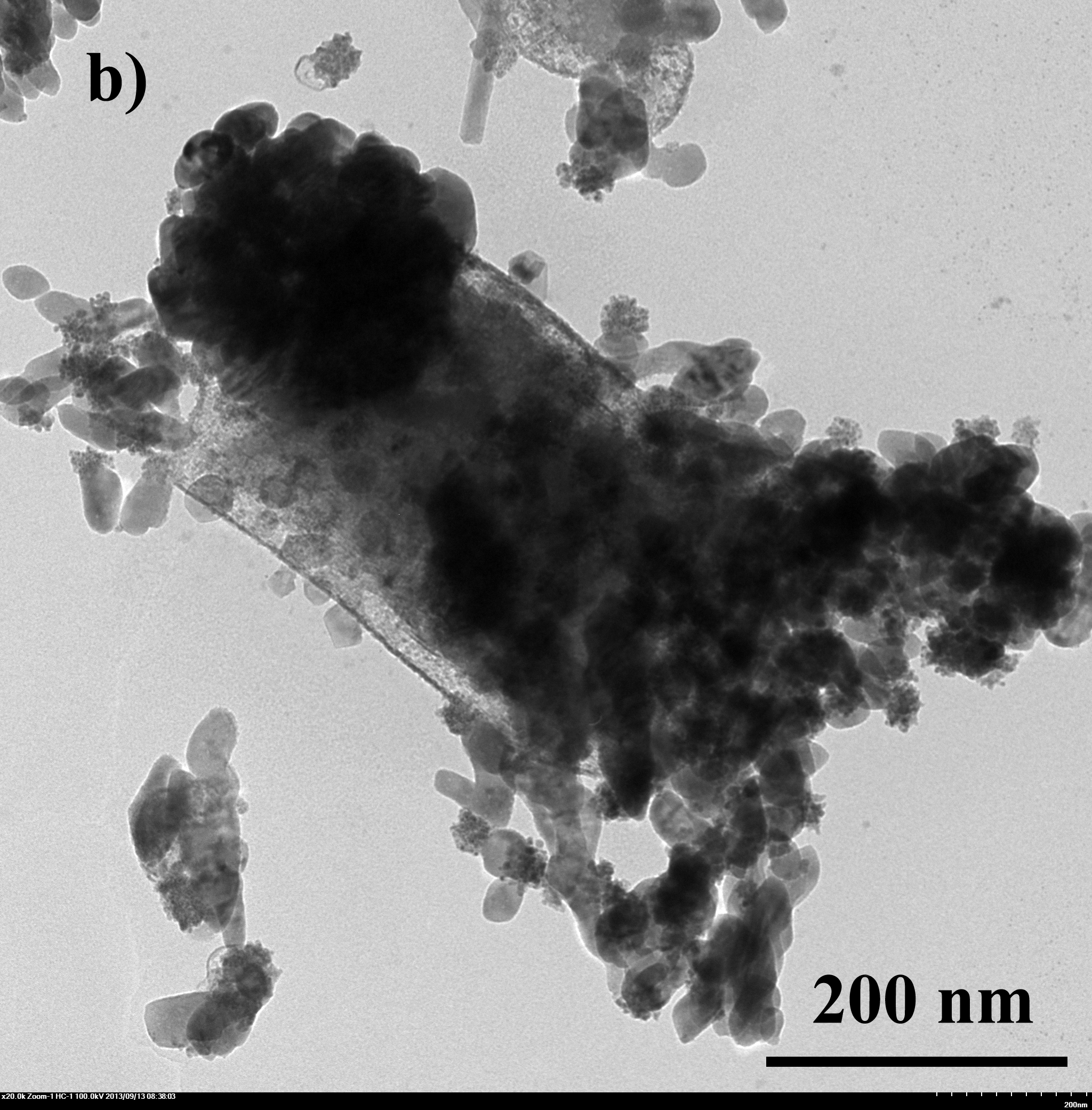
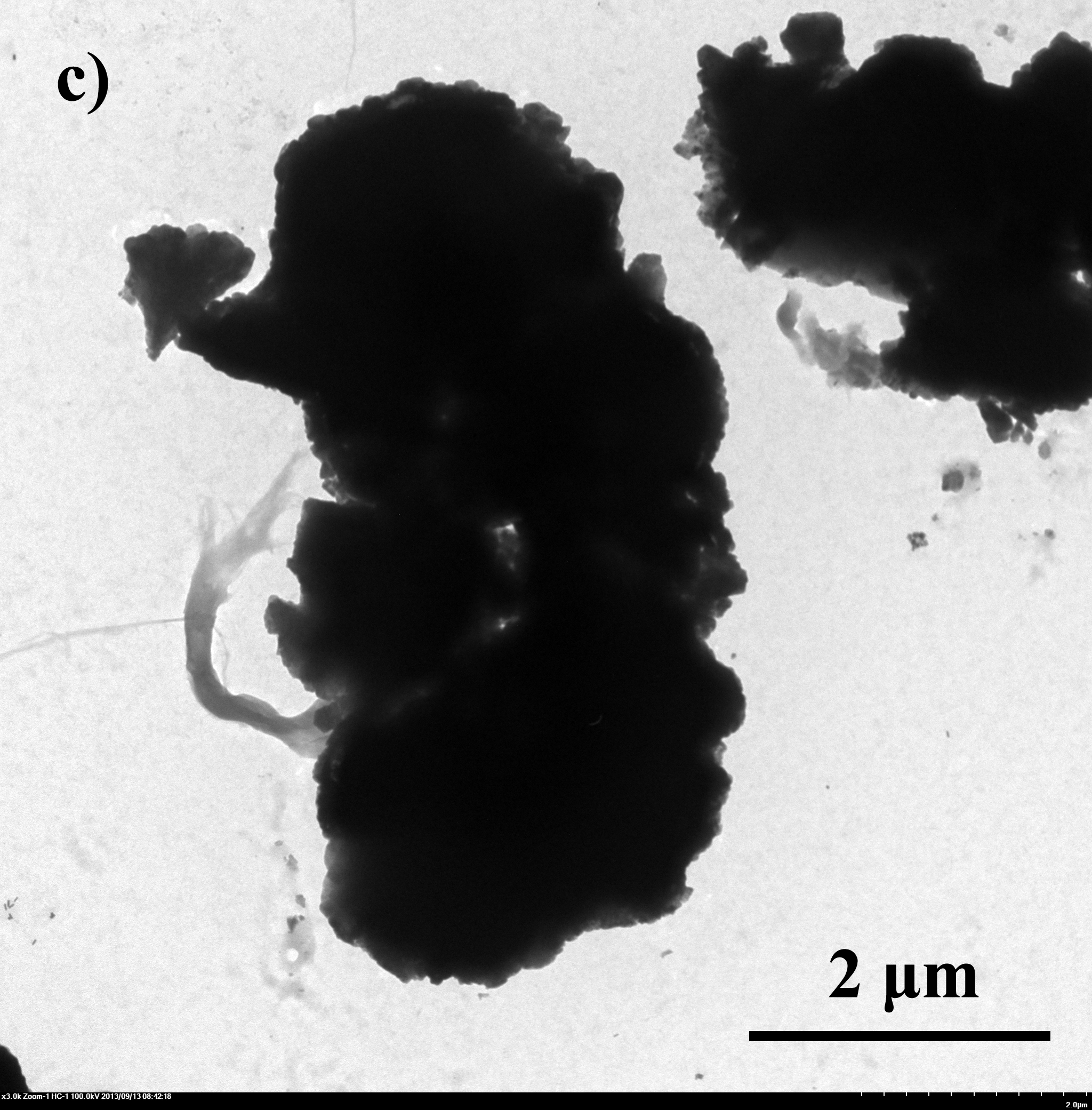
| - | + | Fig.4 TEM image of biominerized ''E.coli'': a)bare ''E.coli''; b) ''E.coli'' with CaCO<sub>3</sub> shell, 0.33M CaCl<sub>2</sub>/0.33M Na<sub>2</sub>CO<sub>3</sub> c) ''E.coli'' with Na<sub>2</sub>HPO<sub>4</sub> shell, 0.05M CaCl<sub>2</sub>/0.05M Na<sub>2</sub>HPO<sub>4</sub> | |
</center> | </center> | ||
Revision as of 08:17, 28 October 2013
Safety Part I : Calcium phosphate and Calcium carbonate shell
Contents |
Overview
Our atrazine killer is designed to move in the plate and in the soil, if applied to eliminate the atrazine in the environment, and thus we need to guarantee that it will not move around and pose potential threat to the environment. Here, we come up with an idea to encapsulate our engineered bacteria with CaCO3/CaP (amorphous calcium phosphate) on the purpose of constraining their movement before using and extending the preservation time.
Background
During our daily life, we can see egg shells everywhere and we know that they are consist of CaCO3. Also, our bones and teeth are made of CaP ((Ca10(PO4)x(OH)y)). In fact, as biocompatible, stable, and nontoxic templates, these two kind of materials already have received increased attention.
It is believed that artificial shells can extend preservation time and protect bacteria from the outside world. Ben Wang et.al has made mineral artificial shells on yeast cells and proved that they would go into G0 state since proliferation of the encased cells is not observed even in the nutritive medium, which means yeast would not grow or die for a long time in a shell.
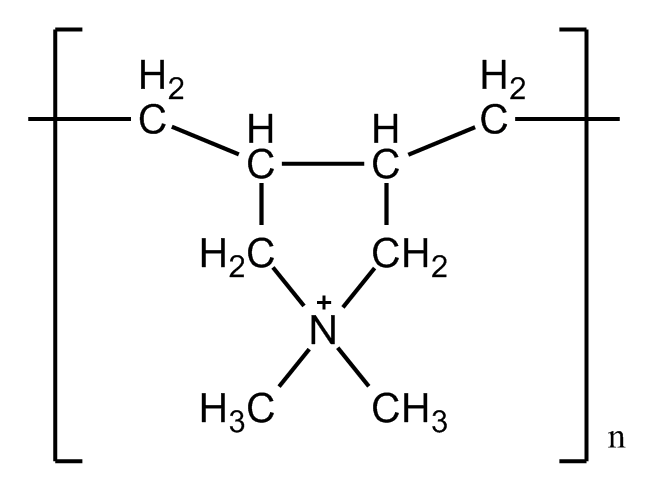
Previous studies also pointed out that the electronic interaction is a key factor in biomineralization, especially the carboxylates(-COOH) and hydroxyl(-OH) or other charged functional groups rich in proteins. Cell surface can also be modified by introducing some functional factors. Polymers are usually been used because of the good capacity for self-assembly. Opposite charged polymers, such as PAH/PSS or PDADMAC/PAH are widely applied because of their good ability to form LBL layers on the surface and provide carboxylates that can bind to Ca2+.
The use of decomposable shell will not only prevent our engineered bacteria from posing potential threat to environment before they are designed to function, but have little negative impact on the viability of the living organism.
Design
We believe that E.coli share a similar character with the yeast cells, since they all have polysaccharide on the surface, which provide negatively charged hydroxyl(-OH) to bind with Ca2+. Thus we designed a calcium phosphate shell. In comparison, we used calcium carbonate shell to see the result. Also, we tried polymers to better form the shell.
Fig.1 Molecular structure of lipopolysaccharide
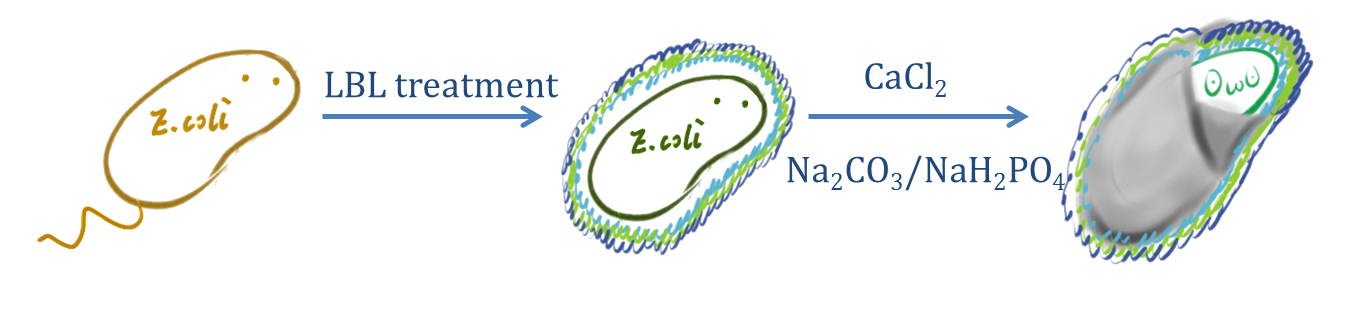
CaCO3/CaP spontaneously precipitates by mixing of two concentrated solutions of calcium and carbonate/phosphate. The precipitation leads to small, spherical and non-aggregated amorphous nano-precipitates, instantly formed upon mixing saturated salt solutions like CaCl2 and Na2CO3/Na2HPO4.
Fig.2 Schematic diagram of the preparation of Calcium phosphate/carbonate shell
Fig.3 Schematic diagram of the preparation of LBL treated polymer shell
Characterization
E.coli with artificial shells were viewed under SEM and TEM. From fig 4 we can tell that the artificial shells have successfully encapsuled E.coli.
Fig.4 TEM image of biominerized E.coli: a)bare E.coli; b) E.coli with CaCO3 shell, 0.33M CaCl2/0.33M Na2CO3 c) E.coli with Na2HPO4 shell, 0.05M CaCl2/0.05M Na2HPO4
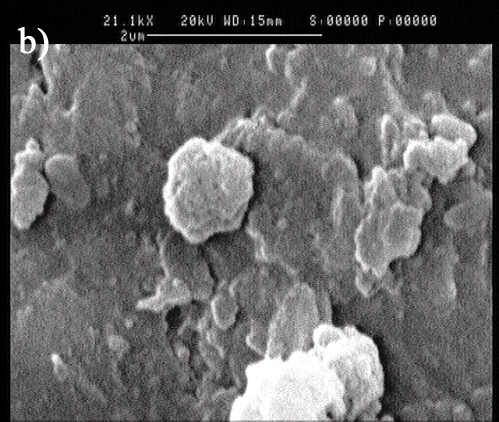
When capsuled by LBL multilayer and mineral, a spherical calcium phosphate has been formed.
Figure 4. TEM and SEM image of multilayer biominerized E.Coli: a)TEM image; b) SEM image
Also, EDTA were added after encapsulation to test whether it would affect the bacteria’s viability. The GFP detected by fluorescence microscope showed that most bacteria did not die after the shell formation and EDTA treatment procedure.
Figure 5. E.Coli GFP expression after shell formation and EDTA treatment procedure under fluorescence microscope
Supporting imformation
Protocol of Encapsulation of E.coli using CaCO3
- 1 mL of overnight culture are added to a sterile reaction tube and centrifuged for 1 min at 12000 rpm.
- The cells are washed 3 times with 0.33M CaCl2
- Suspend the cells in 5 mL of 0.33M CaCl2 solution and incubate them under gentle stirring for 15 min.
- A 5mL aliquot of 0.33M Na2CO3 is added under vigorous stirring for 30s.
- Keep the whole suspension under stirring for 30 min for the precipitation of the CaCO3 microcapsules.
Protocol of Encapsulation of E.coli using ACP
- 1 mL of overnight culture are added to a sterile reaction tube and centrifuged for 1 min at 12000 rpm.
- The cells are washed 3 times with 0.05M CaCl2
- Suspend the cells in 5 mL of 0.05M CaCl2 solution and incubate them under gentle stirring for 15 min.
- A 5 mL aliquot of 0.03M Na2HPO4 is added under vigorous stirring at the speed of 3 drops per minutes.
- Keep the whole suspension under stirring for 30 min for the precipitation of the ACP microcapsules.
- To release the cells from the calcium capsules, the capsules are dispersed in 0.25M EDTA (pH=8) solution under constant shaking for 30min and afterwards washed twice with 0.25M EDTA(pH=8) and twice with 0.05M NaCl.
Reference
[1] Yeast Cells with an Artificial Mineral Shell: Protection and Modification of Living Cells by Biomimetic Mineralization. Ben Wang et.al. Angew. Chem. 2008, 47 , 3560–3564
[2] Encapsulation of LivingE. coli Cells in Hollow Polymer Microspheres of Highly Defined Size. Jennifer Flemke et.al. Biomacromolecules. 2013, 14, 207 - 214
 "
"