Team:NYMU-Taipei/Modeling/ModSensors
From 2013.igem.org
| Line 1: | Line 1: | ||
{{:Team:NYMU-Taipei/Header}} | {{:Team:NYMU-Taipei/Header}} | ||
=Sensors= | =Sensors= | ||
| - | ==Function of the parts | + | ==Function of the parts== |
TrxC promoter is an OxyR-activated promoter. We use TrxC promoter as a sensor/ switch to have the whole circuit opened when Nosema exists, and closed when Nosema is killed. | TrxC promoter is an OxyR-activated promoter. We use TrxC promoter as a sensor/ switch to have the whole circuit opened when Nosema exists, and closed when Nosema is killed. | ||
| Line 12: | Line 12: | ||
[[Image:NYMU_ros oxyr complex.png|center]] | [[Image:NYMU_ros oxyr complex.png|center]] | ||
| - | ==The purpose of this | + | ==The purpose of this model== |
#To know the lag time between sensing the invasion and the production of the killing protein. | #To know the lag time between sensing the invasion and the production of the killing protein. | ||
| Line 85: | Line 85: | ||
[[know more4]] | [[know more4]] | ||
| - | ==Results | + | ==Results== |
[[Image:NYMU_without Nosema invasion.png|center]] | [[Image:NYMU_without Nosema invasion.png|center]] | ||
Revision as of 15:52, 28 October 2013


Contents |
Sensors
Function of the parts
TrxC promoter is an OxyR-activated promoter. We use TrxC promoter as a sensor/ switch to have the whole circuit opened when Nosema exists, and closed when Nosema is killed.
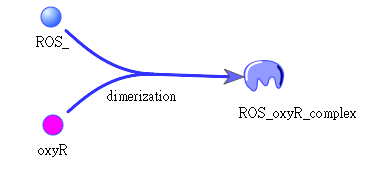
When Nosema enters the bees, it will trigger bees’ ROS (Reactive Oxygen Species) production, which in turn launches E. coli’s OxyR production. After that ROS and OxyR will form a complex and binds to TrxC promoter. To increase the sensitivity of this switch, we add an additional circuit expressing OxyR regulated by a constitutive promoter to enhance the basal OxyR expression in E. coli.
The picture shows how oxyR and ROS form a complex:
The purpose of this model
- To know the lag time between sensing the invasion and the production of the killing protein.
- The minimal Oxidative stress that can activate the switch by choosing the proper constitutive promoter for boosting OxyR's concentration
- To know the relation between ROS input and the TrxC promoter open strength.
- To see to which degree will TrxC promoter influence the production of LuxI, LuxR, and TetR, which will influence the second and the third circuit.
It is assumed that the concentration of ROS in bees is relative to the severity of Nosema infection, and that OxyR is so abundant that once ROS appears, it will soon bind to ROS to form OxyR* and reach equilibrium.
Equation1:
PoPSconstitutive = promoter strength of constitutive promoter (J23102)
N = number of plasmid in a single cell
V = volume of a cell
The aim of the equation is to know the production rate of mRNAOxyR, and choose the proper constitutive promoter for boosting OxyR's concentration.
Equation2:
RBS = binding site strength
KdegOxyR = degrading constant of OxyR
The aim of the equation is to know the production rate of OxyR and when it can reach the concentration activating TrxC promoter, which can in turn be deduced to get the lag time between sensing the invasion and the production of the killing protein.
Equation3:
KOxyR* = constant rate of ROS+OxyR→OxyR*
The aim of the equation is to know the concentration of OxyR* given the concentration of ROS and OxyR, which will know the relation between ROS input and the TrxC promoter open time.
Equation4:
PoPSconstitutive = promoter strength of constitutive promoter
N = number of plasmid in a single cell
V = volume of a cell
The aim of the equation is to know how TrxC promoter strength (in PoPS) influences the production of TetR, LuxI, and LuxR.
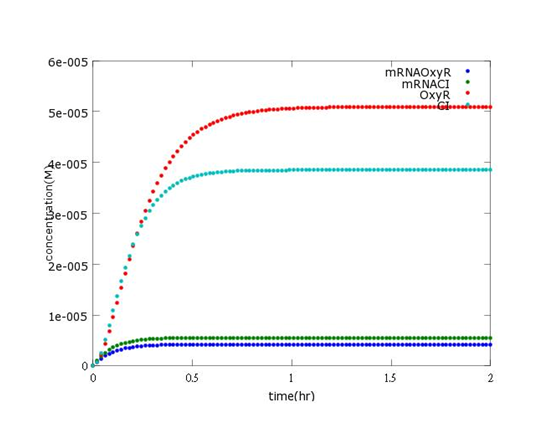
Results
This picture predicts the buildup of concentration of mRNAOxyR, mRNACI, OxyR, CI will reach in a newborn Bee. coli (without Nosema invasion).
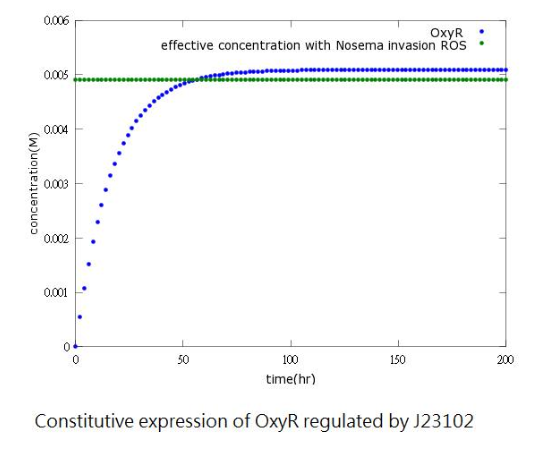
Since naturally produced oxyR in Bee.coli (our E.coli) is not sufficient, we add a constitutive promoter before the oxyR-producing gene to increase oxyR concentration to reach the effective level (0.005M). The picture shows that after adding constitutive promoter, oxyR concentration can reach the effective level to activate ROS/oxyR complex-induced promoter 50 hours after Nosema infection.
Discussion:
The results shown in killing protein and ethanol production means that the adjusted OxyR concentration is high enough to swiftly form a complex after ROS is heighten, therefore turning on the switch.
In reality, the final centration of OxyR can reach much more higher than we had expected after a longer period of time. The inaccuracy will be discussed below.
The reason why the actual result of OxyR concentration differing from the expected one may be that we did not take the negative feedback of OxyR production.
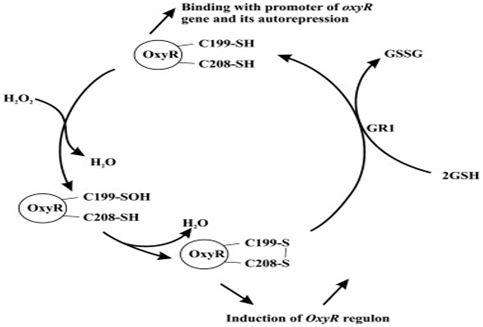
This picture shows OxyR cycle in E. coli:
The top molecule is reduced form of OxyR, whose Cys199 to sulfoxide will be oxidized by hydrogen peroxide (the oxidative reagent as well as a kind of ROS).
After the water exclusion and disulfide bond formation, the reduced form of oxy turns into the oxidized one. Oxidized OxyR is an activator of target promoter, which stimulates downstream gene expression. However, Oxidized form of OxyR protein is transformed into reduced OxyR protein (inactivated) by thioredoxin reductase (GSH). Reduced OxyR protein (the top molecule)provides the autorepression of OxyR gene (a negative feedback).
Parameters:
| Model | Parameter | Description | Value | Unit | Reference |
|---|---|---|---|---|---|
| Sensor | KdegOxyR | OxyR degrading rate | 107 | M-1 x min-1 | Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol—disulfide status |
| KdOxyR* | Dissociation constant of OxyR* | 10-7.33 | M | ||
| KOxyR | OxyR producing rate constant | 5.012 x 1014 | X | ||
| nOxyR* | Hill coefficient of OxyR* | Ranging from 0.75~3.5,depends on what kind of ROS it react with | X | OxyR: A Molecular Code for Redox-Related Signaling | |
| N | Copy number | 887(cells containing 25% plasmid bearing cells) | Single piece |
|
 "
"