Team:Evry/Sensor
From 2013.igem.org
| Line 34: | Line 34: | ||
<p align="center"> | <p align="center"> | ||
<b>Fig. 1</b> Construction of an iron-responsive genetic element by fusing a Fur-regulated promoter with a reporter gene. | <b>Fig. 1</b> Construction of an iron-responsive genetic element by fusing a Fur-regulated promoter with a reporter gene. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</p> | </p> | ||
| Line 66: | Line 40: | ||
<tr> | <tr> | ||
<th align="center"> | <th align="center"> | ||
| - | <b> | + | <b>Name</b> |
</th> | </th> | ||
<th align="center"> | <th align="center"> | ||
| - | <b> | + | <b>Figure</b> |
</th> | </th> | ||
<th align="center"> | <th align="center"> | ||
| - | <b> | + | <b>Description</b> |
</th> | </th> | ||
</tr> | </tr> | ||
| Line 140: | Line 114: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| + | |||
| + | <p align="center"> | ||
| + | <b>Table 1</b> Genetic elements used to make iron-responsive sensors. | ||
| + | </p> | ||
| + | |||
| + | |||
<p> | <p> | ||
| - | < | + | These biosensors respond to ambient iron by using the <a href="https://2013.igem.org/Team:Evry/Project_FUR">Fur system</a> to repress the reporter gene placed downstream the promoter. |
</p> | </p> | ||
| + | |||
| + | <h2>Caracterisation of the iron-responsive biosensors</h2> | ||
| + | |||
| + | <p> | ||
| + | As shown in figure 2, in our construction, sfGFP is placed downstream the Fur binding site. It means that in iron starvation sfGFP should be expressed and in high concentration of iron it should be repressed. | ||
| + | </p> | ||
| + | |||
| + | <br/> | ||
| + | |||
| + | <div align='center'><img src="https://static.igem.org/mediawiki/2013/1/12/ColiSensor.png" width="75%"/></div> | ||
| + | <p align="center"> | ||
| + | <b>Fig. 2</b> Diagram of our genetic iron sensor. Iron binds the Ferric Uptake Regulator (Fur) to form a complex with high affinity for the Fur box in the promoter, here shown as the aceB promoter. Once the iron-Fur complex is bound to the promoter, it represses transcription of the target gene GFP. GFP expression is thus negatively correlated with iron availability. | ||
| + | </p> | ||
| + | <br> | ||
| + | <br> | ||
| + | <div align='center'><img src="https://static.igem.org/mediawiki/2013/7/7b/P1-GFP.png" width="75%"/></div> | ||
| + | |||
| + | <div align='center'><img src="https://static.igem.org/mediawiki/2013/f/f3/P1-Nat_Prom.png" width="58%"/></div> | ||
| + | |||
| + | <p> | ||
| + | <b>Fig 2</b> Construction of an iron-responsive genetic element by fusing a Fur-regulated promoter with a reporter gene. Promoter-reporter fusions were made with flanking restriction sites that are compatible with Biobrick-based cloning. | ||
| + | </p> | ||
| + | |||
| + | |||
| + | |||
Revision as of 19:47, 28 October 2013
Iron Sensor

Construction of the iron-responsive biosensors
E. coli's genome is composed of many Fur binding site. Based on a genome study, we identified 4 promoters which are controled by the FUR protein.
- AceB promoter - (BBa_K1163102)
- Fes promoter - (BBa_K1163108)
- FepA promoter - (BBa_K1163105)
- yncE promoter - (BBa_K1163111)
Using PCR on E. coli genome, we extracted these four promoters. We constructed iron-responsive biosensors by combining 3 genetic parts: an E. coli promoter with a Ferric Uptake Regulator (Fur) binding site, a fluorescent reporter (sfGFP), and a transcriptional terminator (see Figure 1 below). Promoter-reporter fusions were made with flanking restriction sites that are compatible with Biobrick-based cloning.


Fig. 1 Construction of an iron-responsive genetic element by fusing a Fur-regulated promoter with a reporter gene.
| Name | Figure | Description |
|---|---|---|
|
E. coli promoter with Fur binding site |

|
iron-Fur complex binds promoter to repress expression |
|
sfGFP |

|
Fluorescent reporter gene |
|
Terminator |

|
terminator to stop transcription |
|
Plasmid |

|
Biobrick-compatible plasmid backbone |
Table 1 Genetic elements used to make iron-responsive sensors.
These biosensors respond to ambient iron by using the Fur system to repress the reporter gene placed downstream the promoter.
Caracterisation of the iron-responsive biosensors
As shown in figure 2, in our construction, sfGFP is placed downstream the Fur binding site. It means that in iron starvation sfGFP should be expressed and in high concentration of iron it should be repressed.
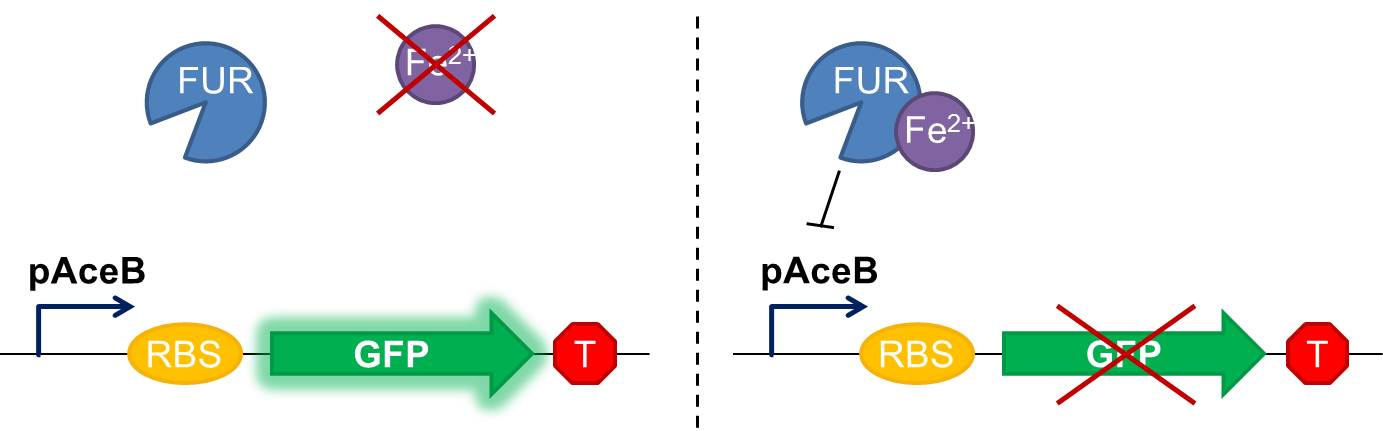
Fig. 2 Diagram of our genetic iron sensor. Iron binds the Ferric Uptake Regulator (Fur) to form a complex with high affinity for the Fur box in the promoter, here shown as the aceB promoter. Once the iron-Fur complex is bound to the promoter, it represses transcription of the target gene GFP. GFP expression is thus negatively correlated with iron availability.


Fig 2 Construction of an iron-responsive genetic element by fusing a Fur-regulated promoter with a reporter gene. Promoter-reporter fusions were made with flanking restriction sites that are compatible with Biobrick-based cloning.
 "
"













