Team:Paris Saclay/Notebook/August/14
From 2013.igem.org
Notebook : August 14
Lab work
A - Aerobic/Anaerobic regulation system
Objective : obtaining ...
1 - Digestion of Bba_K1155004,Bba_K1155005, Bba_K1155006 by EcoRI/SpeI
Nadia
- Buffer FD : 2µL
- H2O : 6µL
- DNA : 10µL
- SpeI FD : 1µL
- EcoRI FD : 1µL
2 - Electrophoresis to check the digestion of Bba_K1155004,Bba_K1155005, Bba_K1155006 by EcoRI/SpeI
Anaïs, Nadia
Expected sizes :
- PSB1C3 : 2070kb
- NarK, NarG, NirB : 200kb
|
We obtained NarK, NarG, NirB fragments at the right size but in very few quantity. We do it again but this time we will use more quantity of enzymes AND ADN ?!?????????? |
3 - Digestion of Bba_K1155000, Bba_K1155004,Bba_K1155005, Bba_K1155006 by SpeI and EcoRI/SpeI
Anaïs, Nadia
- Digestion by SpeI :
- Buffer : 2µL
- SpeI : 2µL
- ADN : 15µL
- H20 : 1µL
- Digestion by EcoRI and SpeI :
- Buffer : 3µL
- SpeI : 2µL
- EcoRI : 2µL
- ADN : 20µL
- H20 : 3µL
4 - Electrophoresis to check the digestion of Bba_K1155000, Bba_K1155004,Bba_K1155005, Bba_K1155006 by SpeI and EcoRI/SpeI
Anaïs, Nadia
Expected sizes :
- Pfnr in PSB1C3 : ...
- Nar K in PSB1C3, NarG in PSB1C3, NirB in PSB1C3 : ...
- PSB1C3 : 2070kb
- NarK, NarG, NirB : 200kb
- Pfnr : ...
|
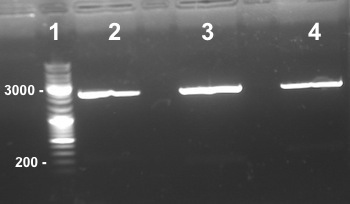
Well 1 : we have done one digestion so we have to obtain one fragment, we have two stripes, the digestion of Bba_K1155000 by SpeI wasn't good. Well 2, 3, 4, 6, 7 : we obtain NarG, NarK and NirB in PSB1C3 and Pfnr, NarK fragments at the right size, we can purify it. Well 8, 9 : we have done two digestions so we have to obtain two fragments, we have only one stripe, the digestion of Bba_K1155005 and Bba_K1155004 by EcoRI/SpeI wasn't good. |
5 - Gel purification of the digestion of Bba_K1155000 and Bba_K1155006 by EcoRI/SpeI and Bba_K1155004, Bba_K1155005 and Bba_K1155006 by SpeI
Anaïs, Nadia
Protocol : Gel purification
| [[]] |
Picture : Electrophoresis gel of the digestion of Bba_K1155000, Bba_K1155004,Bba_K1155005, Bba_K1155006 by SpeI and EcoRI/SpeI
|
Nanodrop :
- Pfnr : 13.7ng/µL
- NarK : 27.9ng/µL
- NarK in PSB1C3 : 87.1ng/µL
- NarG in PSB1C3 : 39.2ng/µL
- NirB in PSB1C3 : 37.7ng/µL
|
CONCLUSION |
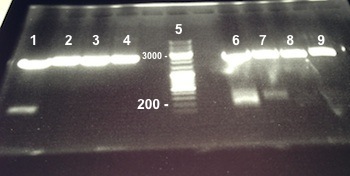
6 - Gel purification of the digestion of Bba_K1155003 and Bba_K1155007 by XBaI/PstI
Nadia
| [[]] |
Picture : Electrophoresis gel of the digestion of Bba_K1155003 and Bba_K1155007 by XBaI/PstI
|
A - Aerobic/Anaerobic regulation system / B - PCB sensor system
Objective : obtaining FNR and BphR2 proteins (Gibson assembly)
1 - Gibson assembly
PRECISIONS !!!!!!!!!!!!!!!!!!!!!!
2 - Electrophoresis of PCR products : BphR2 Part I
Damir
| [[]] |
|
| [[]] |
|
Expected sizes :
- BphR2 Part I : 178kb
|
On the first gel, all deposits disappear so we did the electrophoresis again. On the second gel, we didn't obtain stripes at the good size. We do the PCR again using new quantities and a new PCR program. |
3 - PCR of BphR2 Part I
Damir
Used quantities :
- Oligo 54F : 2µL
- Oligo 55R : 2µL
- DNA : 1µL
- Buffer Phusion : 10µL
- dNTP : 1µL
- Phusion : 1µL
- DMS9 : 2µL ??????????????????????????????????
- H2O : 31µL
PCR program :
[[]]
 "
"